views
If you know the formula of an ionic compound and are trying to figure out its English name, read about naming ionic compounds instead.
Writing Formulas for Simple Binary Compounds
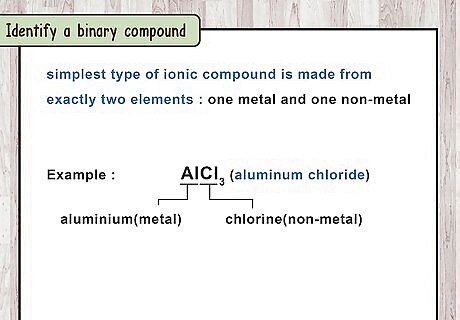
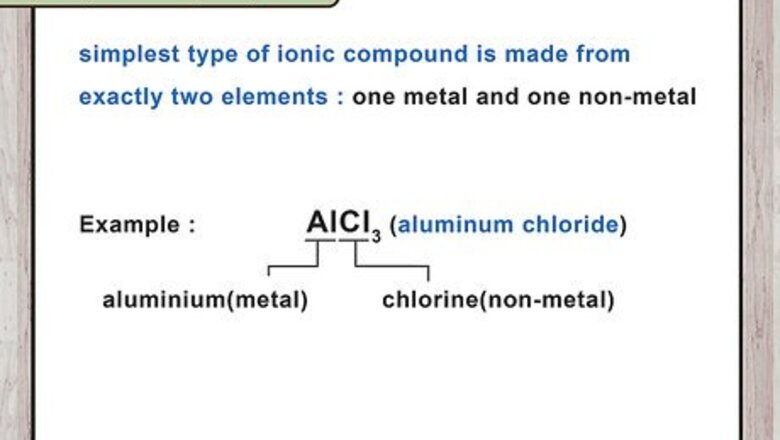
Identify a binary compound. The simplest type of ionic compound is made from exactly 2 elements, 1 metal and 1 non-metal. Their name is always written as 2 element names, plus the -ide suffix attached to the second name. Examples of simple binary ionic compounds include potassium oxide and sodium phosphide. If the "-ide" suffix doesn't follow a single element name, see instructions for polyatomic ions. For example, "oxide" is a simple oxygen ion, but "hydroxide" and "peroxide" are polyatomic.
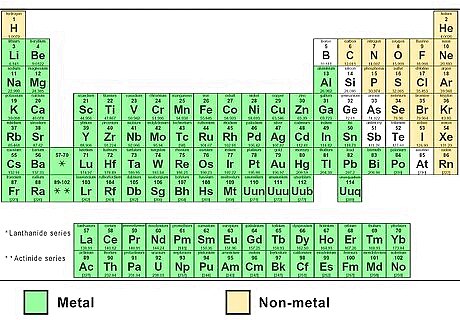
Look up the 2 elements on a periodic table. The first word of the name refers to the metal ion (the positively charged cation). You can find this element on the left side of the periodic table. The second word, ending in -ide, refers to a non-metal ion (the negatively charged anion). Find this on the right side of the periodic table . For example, potassium oxide is a combination of potassium (chemical symbol K, atomic number 19) and oxygen (O, atomic number 8). Notice that the -ide ending is not part of the element name. You're just looking for an element with the same beginning (in this case ox-).
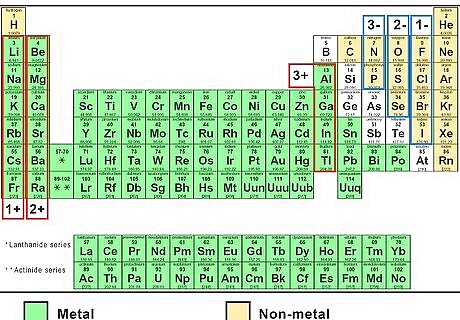
Find the charge of each ion. In these simple compounds, it is easy to predict the charge of each ion. Each element in a particular column of the periodic table always forms an ion with the same charge: The Group 1 elements Li, Na, K, Rb, and Cs gain a charge of 1+ (written simply as +). The Group 2 elements Mg, Ca, Sr, and Ba gain a charge of 2+. The Group 13 element Al gains a charge of 3+. The Group 15 elements N and P gain a charge of 3-. The Group 16 elements O and S gain a charge of 2-. The Group 17 elements F, Cl, Br, and I gain a charge of 1- (written as -).
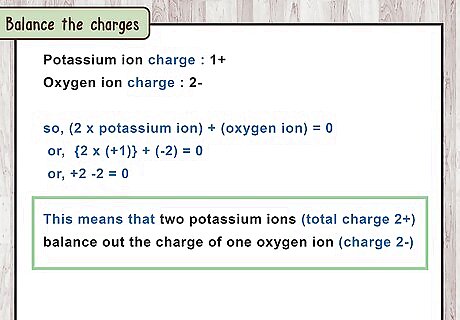
Balance the charges. It’s crucial to balance the charges in order to write ionic compounds correctly. Electrical forces hold together ionic compounds, pulling the positive and negative ions together. Taken as a whole, the ionic compound is electrically neutral, meaning it has a total charge of zero. (If it had a different charge, it would pull in another atom.) Find the number of atoms of each element that combine to "cancel out" each other's charge and make a neutral compound. For example, potassium oxide is made up of potassium ions K + {\displaystyle K^{+}} K^{+} and oxygen ions O 2 − {\displaystyle O^{2-}} O^{{2-}}. This means that 2 potassium ions (total charge 2+) balance out the charge of 1 oxygen ion (charge 2-). Here's a shortcut: the first ion's charge (ignoring + or -) tells you the number of atoms of the second ion, and vice versa. For example, aluminum fluoride is made of A l 3 + {\displaystyle Al^{3+}} Al^{{3+}} and F − {\displaystyle F^{-}} F^{{-}} ions. The charge of A l 3 + {\displaystyle Al^{3+}} Al^{{3+}} is 3, so there are 3 F − {\displaystyle F^{-}} F^{{-}} atoms. The charge of F − {\displaystyle F^{-}} F^{{-}} is 1, so there is 1 A l 3 + {\displaystyle Al^{3+}} Al^{{3+}} atom.
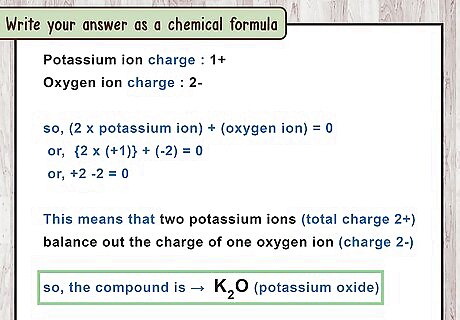
Write your answer as a chemical formula. Write the 2 chemical symbols in the same order they appear in the compound's name (metal then nonmetal). After each chemical symbol, write the number of atoms as subscript ( l i k e t h i s {\displaystyle _{like\ this}} _{{like\ this}}). If there is exactly 1 atom of an element, you do not need a number. There is no need to write the charges. For example, potassium oxide has 2 potassium atoms and 1 oxygen atom. The chemical formula is K 2 O {\displaystyle K_{2}O} K_{{2}}O. Aluminum fluoride has 1 aluminum atom and 3 fluorine atoms. The chemical formula is A l F 3 {\displaystyle AlF_{3}} AlF_{{3}}.

Simplify if possible. Ionic compound formulas are always written in the empirical formula, meaning they’re written with the minimum number of atoms required. If you could balance out the charges with fewer atoms, rewrite the formula. This is the same process as reducing fractions. For example, barium sulfide is made of B a 2 + {\displaystyle Ba^{2+}} Ba^{{2+}} and S 2 − {\displaystyle S^{2-}} S^{{2-}} ions. Using the shortcut above, the barium ion's charge (2) is equal to the number of sulfur ions, and the sulfur ion's charge (2) is equal to the number of barium ions. This gives us the formula B a 2 S 2 {\displaystyle Ba_{2}S_{2}} Ba_{{2}}S_{{2}}. However, you don't need this many atoms to balance the charges. Write the 2 numbers as a fraction and simplify: 2 b a r i u m a t o m s 2 s u l f u r a t o m s = 1 1 {\displaystyle {\frac {2\ barium\ atoms}{2\ sulfur\ atoms}}={\frac {1}{1}}} {\frac {2\ barium\ atoms}{2\ sulfur\ atoms}}={\frac {1}{1}}, so the correct formula is B a S {\displaystyle BaS} BaS.
Binary Compounds with Transition Metals
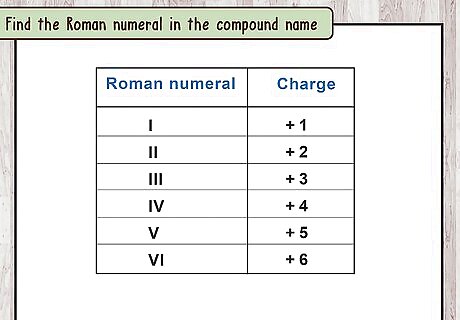
Find the Roman numeral in the compound name. Most elements in the "d-block" of the periodic table (groups 3 through 12) are considered transition metals. Unlike other elements, they can form more than one type of ion. When a transition metal is part of an ionic compound, a Roman numeral is included to specify which ion is involved. For example, copper sometimes forms an ion with a charge of +1, and sometimes with a charge of +2. Copper (I) chloride and copper (II) chloride are 2 different ionic compounds. The Roman numeral tells you which copper ion is part of the compound.
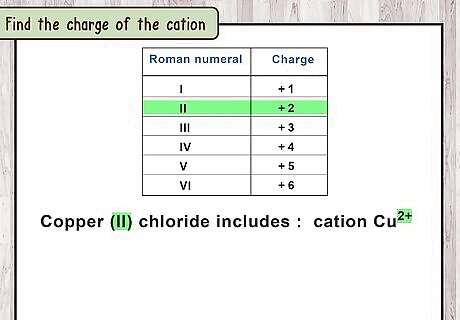
Find the charge of the cation. The first word of the compound name represents the metal ion, or cation. This is always positively charged. Just add the Roman numeral as a positive charge to the element name: For example, copper (II) chloride includes the cation C u 2 + {\displaystyle Cu^{2+}} Cu^{{2+}}, since II is the Roman numeral for 2.
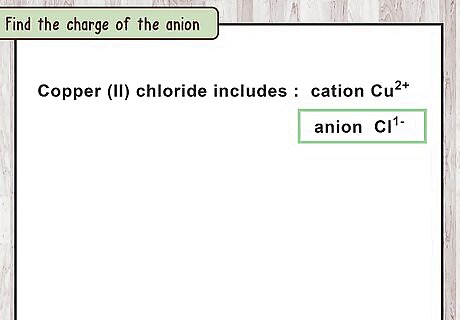
Find the charge of the anion. The second word of the compound name represent the anion. This is the negatively charged ions that form from non-metallic elements. Each element of this type only forms 1 ion. To find the charge of that ion, look it up in a textbook or chemistry website, or memorize the group rules. For example, chloride is the name for a chlorine ion. Chlorine, like similar group 17 elements, forms ions with a charge of negative one. This is written as C l − {\displaystyle Cl^{-}} Cl^{{-}}.
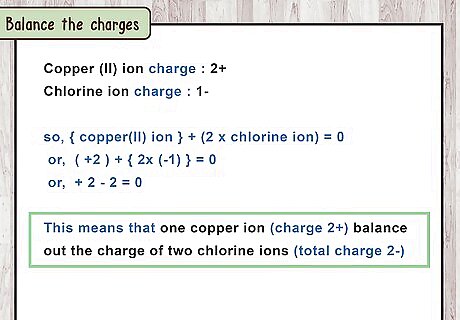
Balance the charges. This process is the same as for any ionic compound. The total positive charge from the cations always balances out the total negative charge from the anions. The net charge of the ionic compound is always zero. To balance out the 2+ charge from a C u 2 + {\displaystyle Cu^{2+}} Cu^{{2+}} ion, you need 2 C l − {\displaystyle Cl^{-}} Cl^{{-}} ions (2 ions x -1 charge per ion = -2). This makes an ionic compound with a net charge of +2 -2 = 0.
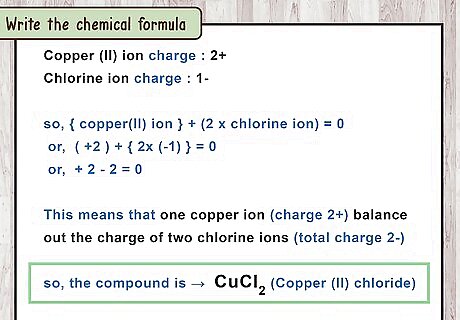
Write the chemical formula. As always, the number of atoms is written as a subscript after the element name. You do not need to write the charges in the final formula: Copper (II) chloride has one copper atom and two chlorine atoms. The formula is C u C l 2 {\displaystyle CuCl_{2}} CuCl_{{2}}.
Compounds with Polyatomic Ions
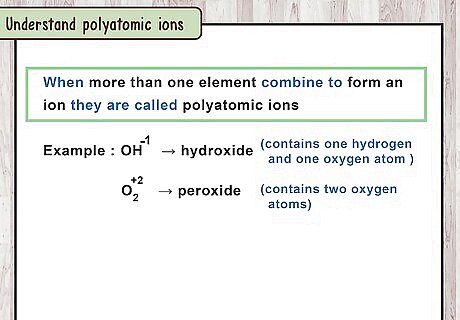
Understand polyatomic ions. Sometimes, more than one element can combine to form an ion, which can then bond with a different ion to form a compound. For example, O H − 1 {\displaystyle OH^{-1}} OH^{{-1}} is a polyatomic ion made from oxygen and hydrogen ions. Each polyatomic ion has its own special name. O H − 1 {\displaystyle OH^{-1}} OH^{{-1}} is called "hydroxide." O H − 1 {\displaystyle OH^{-1}} OH^{{-1}} is not an ionic compound, because it does not have a net charge of zero. It is a single ion, which can combine with ions of the opposite charge. Some elements can form polyatomic ions by themselves. For example, "peroxide" is the O 2 2 − {\displaystyle O_{2}^{2-}} O_{{2}}^{{2-}} ion. It contains 2 oxygen atoms, but has a total charge of -2. (This is different than oxide, the "standard" oxygen ion, which has one atom with a charge of -2.)
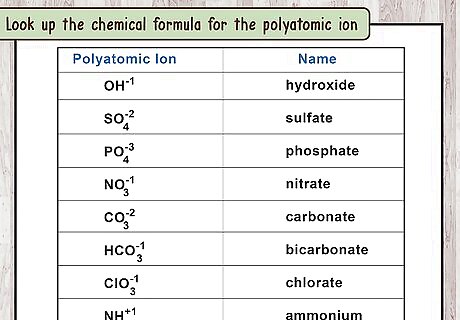
Look up the chemical formula for the polyatomic ion. The second word in the ionic compound's name refers to the polyatomic ion. Look up this word (not the full compound name) to find the formula for this polyatomic ion. You can also memorize a few common polyatomic ions: Cyanide: C N − {\displaystyle CN^{-}} CN^{{-}} (One carbon, one nitrogen, total charge of -1) Hydroxide: O H − {\displaystyle OH^{-}} OH^{{-}}. Nitrate: N O 3 − {\displaystyle NO_{3}^{-}} NO_{{3}}^{{-}} (One nitrogen, three oxygen, total charge of -1) Nitrite: N O 2 − {\displaystyle NO_{2}^{-}} NO_{{2}}^{{-}} Peroxide: O 2 2 − {\displaystyle O_{2}^{2-}} O_{{2}}^{{2-}} Sulfate: S O 4 2 − {\displaystyle SO_{4}^{2-}} SO_{{4}}^{{2-}} Sulfite: S O 3 2 − {\displaystyle SO_{3}^{2-}} SO_{{3}}^{{2-}}

Find the charge of the other ion. The other ion in the compound is usually a simpler, one-atom ion. You can find its charge the same way you would in any problem. For example, magnesium hydroxide contains a magnesium cation. Magnesium is a group 2 element that forms the ion M g 2 + {\displaystyle Mg^{2+}} Mg^{{2+}}.

Balance the charges of the 2 ions. All ionic compounds have a total net charge of zero. Find the minimum number of positive and negative ions that balance each other's charges perfectly. Remember that the polyatomic ion is a single ion, and cannot be divided into parts. For example, to balance O H − 1 {\displaystyle OH^{-1}} OH^{{-1}} and M g 2 + {\displaystyle Mg^{2+}} Mg^{{2+}} , compare the charges. It takes two -1 charges to balance out one 2+ charge. This means there are two hydroxide ions and one magnesium ion.

Write the formula. To show that there are multiple polyatomic ions, enclose that section of the formula in parentheses. Write the subscript showing the number of atoms after the close parenthesis. In the case that more than one of a certain polyatomic ion is necessary to balance the charge, the entire formula for the polyatomic ion is enclosed in parentheses and the numerical subscript is placed outside the parentheses. This shows that the subscript applies to the entire polyatomic ion. For example, magnesium hydroxide has the chemical formula M g ( O H ) 2 {\displaystyle Mg(OH)_{2}} Mg(OH)_{2}. You do not need to include the charges of the ions.



















Comments
0 comment