views
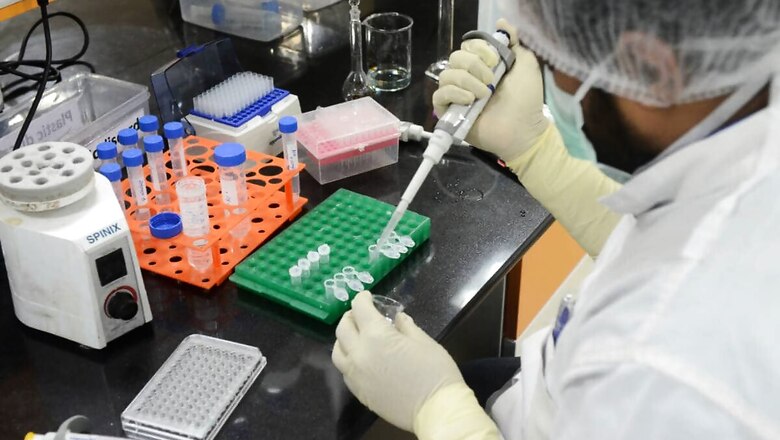
The government on Tuesday refused to wade into the controversy surrounding the allegations of a serious adverse event levelled by a Chennai-based man who participated in the Serum Institute of India’s Covishield vaccine trial. The union health ministry and Indian Council of Medical Research (ICMR) sought to distance themselves from the issue and also refused to comment specifically on the allegation itself, citing a “court case”.
Even as they refused to comment on the specific allegation, ICMR’s director general Dr Balram Bhargava said, “Initial causality assessment, findings did not necessitate stoppage of these trials.”
Responding to questions on the legal notice sent by the Covishield trial volunteer to the Serum Institute of India (SII), union health secretary Rajesh Bhushan said, “We are given to understand that there is a court case, so we would not want to comment on the specifics of the case being considered by the court.”
Contrary to Bhushan’s claim, the Chennai-based 40-year-old volunteer has so far only sent a legal notice to the company.
While avoiding comment on the specific allegation, the union health secretary delved into the procedure to be followed in case serious adverse events occur. Bhushan said that under the framework of new drugs and clinical trial rules, when an adverse event occurs, the principal investigator has to provide information to the Drugs Controller General of India (DCGI). Similarly, an institutional ethics committee, of the hospital where the trials are underway, has to report adverse events to DCGI, he said.
“There is also a data safety monitoring board which monitors the trials and keeps an eye on adverse events. DCGI has to assess information from these multiple sources,” Bhushan said.
According to the legal notice of the Chennai-based volunteer, he suffered from acute neuro encephalopathy and was admitted from October 12 to October 26. SII has refuted the allegations and said that it will seek damages in excess of Rs 100 crore.
“Adverse events do occur with drugs, vaccines and medical treatments. It is the role of the regulator to ascertain or refute causal links after assessing all data. That causal link has to be done by the DCGI once he has got all the papers. This is done purely on a scientific basis and the assessment is done on an objective criteria,” said Dr Bhargava.
When asked about the impact of Serum’s plan to seek damages on volunteerism and confidence in vaccines, Bhushan said that the issue has to be seen in the context of compressed timelines for vaccine development, manufacturing and trials. “In this context, there is a potentiality that commercial interests would dictate certain strategic actions on part of commercial entities. Governments of the day have nothing to do with it,” he said.
He added, “Responsibility of the government, union and state governments, is to educate the people and make them aware of the safety of vaccines, their effectiveness and see that disinformation is countered promptly. It is not the sole responsibility of governments, but of the media, NGOs and vaccine manufacturers. They must collectively educate on vaccine safety and effectiveness.”
Read all the Latest News, Breaking News and Coronavirus News here


















Comments
0 comment