views
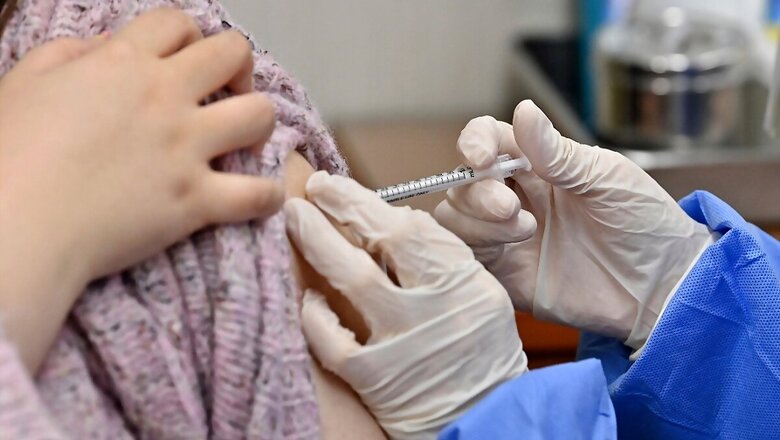
A top Central government panel concluded that Covishield and Covaxin do not increase the risk of blood clotting. The committee submitted its findings after analysing over 400 major adverse reactions reported post-Covid-19 vaccinations.
Member of National Task Force on Covid-19 and advisor to national Adverse Events Following Immunization (AEFI) committee, Dr. NK Arora said that these cases have been analysed and there is no unusual bleeding or clotting manifestations either with Covishield or Covaxin. Of 412 cases, including hospitalisations and deaths, there is no abnormal increase in issues of clotting and bleeding, he reportedly said.
These findings have been submitted to the National Expert Group on Vaccine Administration for Covid-19.
The conclusion comes after several European nations temporarily suspended the rollout of AstraZeneca vaccine over blood clot fears.
Last week, leading EU countries said they would resume the use of the vaccine as the European medical regulator said the jab is “safe and effective”. Pune-based Serum Institute of India’s Covishield has been developed in partnership with the Oxford University and British-Swedish pharmaceutical giant AstraZeneca.
Going forward, a system is in pipeline to ensure a faster turnaround time between the serious issue being reported by states or the Centre and the result of the investigations into them.
The World Health Organization (WHO), however, said that there was no reason to stop using the jab, it stressed that no causal link has been established between the AstraZeneca vaccine and blood clotting.
Meanwhile, a large trial in the US and two South American countries of the Oxford-AstraZeneca vaccine demonstrated a 79 percent efficacy rate at preventing symptomatic Covid-19 and 100 per cent effectiveness in stopping severe disease and hospitalisation, the firm said on March 22.
The company said the panel found “no increased risk of thrombosis or events characterised by thrombosis among the 21,583 participants receiving at least one dose of the vaccine. The specific search for cerebral venous sinus thrombosis (CVST), which is an extremely rare blood clot in the brain, found no events in this trial.”
Read all the Latest News, Breaking News and Coronavirus News here

















Comments
0 comment