views
It was dinner time when she called for help. Before this call, we spoke just twice when she was looking to fill a position in a media house, many years ago.
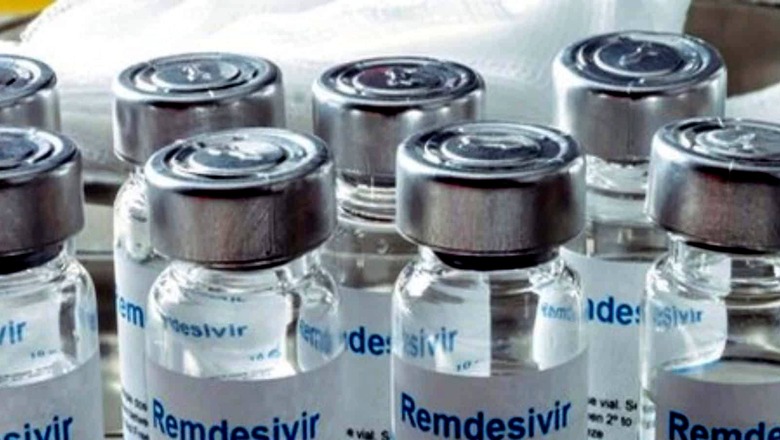
“I need Remdesivir, at any cost, for my father who is admitted to hospital due to Covid-19. I need to save him,” she told me over the phone in a desperate, crying tone.
It was not her first attempt to find the drug, she told me. In fact, she managed to buy one vial of Remdesivir for Rs 30,000 – more than five times of the (then) original MRP of Rs 5500.
But her father’s treating doctor in a reputable hospital in central Delhi informed that the vial is “probably fake”.
“The person who sold it to me had promised that it’s genuine. Now, his number is switched off and our doctor says the vial is not adequately locked.”
Since then, for the next three days, I made multiple calls, but all my attempts failed. From state drug controllers to the officials in top pharma companies – I checked with everyone, but failed to get the vials.
It was the last week of April and we were in the middle of a terrible second wave of Covid. Every possible communication platform – including Twitter, Facebook, Instagram and Whatsapp status messages – was flooded with emotional appeals to find plasma donors or Remdesivir.
Fortunately, her father survived. He started responding to the treatment, which included steroids but not Remdesivir or plasma.
This column is not about her. But it’s about those relatives – son, daughter, wife, sister or be it any other relative – who failed to get Remdesivir and their loved ones failed to survive.
Redeem yourself from the guilt of not being able to save them with Remdesivir. It was an experimental drug, but never the wonder drug.
Dr Pratima Murthy, director of NIMHANS, in October, told me that many relatives who lost their loved ones due to Covid call up their national helpline number to share their grief and inability to save. They are haunted by the memories of jostling for the drug and continue to grieve.
ALSO READ | India Cuts Use of Remdesivir, Tocilizumab, Multivitamins, Steroids in Covid-19 Treatment
National Institute of Mental Health and Neurosciences (NIMHANS) runs a 24×7 national helpline (080-4611 0007) to handle distress calls related to Covid-19.
Once and for all, Remdesivir and plasma were never as effective as we thought.
Here is the proof.
Plasma was dropped from the country’s Covid-19 treatment guidelines after several studies proved its futility. Period.
Now, addressing the elephant in the room – usefulness of Remdesivir.
In India, the ministry of health and family welfare has further curtailed the role of Remdesivir in the treatment of Covid-19.
It can only be given to those patients who require oxygen support, but are not on ventilator support or ECMO. Hence, a very small subset of patients can be given this drug.
The guidelines clearly state that the drug has “no evidence of benefit for treatment more than five days”.
Several doctors believe that the government is “playing safe”.
“They are trying to tick all the boxes,” a doctor told me. “The drug is part of the treatment protocol because till date, there is just one approved anti-viral for Covid-19. They don’t want to use it, but keep it.”
Much before the outbreak of second wave, in November 2020, the World Health Organization issued a conditional recommendation against the use of Remdesivir in hospitalised patients, regardless of disease severity saying “there is currently no evidence that Remdesivir improves survival and other outcomes in these (Covid19) patients”.
The available evidence, based on WHO’s Solidarity trial – suggested no important effect on all parameters that matters – mortality, need for mechanical ventilation, time to clinical improvement, and other patient-important outcomes.
Solidarity trial, conducted in 405 hospitals in 30 countries on patients hospitalised with Covid-19, showed that 2,750 patients on Remdesivir were as likely to die as 2,708 patients on standard care. The drug did not reduce mortality or the time Covid-19 patients take to recover.
In the DisCoVeRy trial, sponsored by the government of France, European Union Commission and others, the drug showed no clinical benefit.
In both, WHO’s Solidarity trial and Recovery trial – some authors, in the past, have received lecture fees or personal fees from Gilead Sciences. The disclaimers showed no one held a significant role in the company.
“If we add data of five clinical trials that included over 7,000 patients with Covid-19, Remdesivir did not reduce risk of death nor need for mechanical ventilation compared with standard of care (routine treatment),” Dr SP Kalantri, professor of Medicine, Mahatma Gandhi Institute of Medical Sciences, Maharashtra, told me.
ALSO READ | Age of Omicron, Immunity War & the Endgame: What We May and May Not Avenge in the New Year
“This should assure the public that it is not a lifesaving drug,” he concluded.
JUST 9 LAKH PATIENTS GOT HOLD OF REMDESIVIR?
The data by IQVIA shows that only 54 lakh vials have been sold, between November 2019-2020 and November 2020-2021. For one patient, six vials are required for the treatment. The general prescription is – 2 vials first day, and then 1 vial a day for four days.
Presuming that everyone managed to complete the full course of medicine, only nine lakh Covid patients ended up taking the drug. In contrast, India has already witnessed more than 3.80 crore Covid cases and around 5 lakh deaths.
As the drug was widely available in the black market, there seems to be no clarity on the actual number of vials sold in India.
Nonetheless, in less than two years, the revenue earned – via legitimate channels – is around Rs 1,500 crore.
STUDIES TO SUPPORT REMDESIVIR
In a recent study published in the New England Journal of Medicine, researchers at the Baylor University Medical Center and affiliated institutions found that a three-day course of Remdesivir lowered the risk of hospitalization by 87.5% in symptomatic, non-hospitalised Covid-19 patients. The study is funded by Gilead Sciences – the originator of the drug.
Also, the manuscript was prepared by a writer who was employed by Gilead Sciences, with input from all authors.
Another latest study led by researchers at Johns Hopkins Medicine supports use of Remdesivir for patients on low-flow oxygen or no oxygen. The study’s lead author, Dr Brian Garibaldi, has received consulting fees from Gilead Life Sciences Inc – the study clarifies in the column under conflict of interest disclosures. Also, he is a member of the US FDA’s Pulmonary-Asthma Drug Advisory Committee. US FDA is the agency that made Remdesivir a wonder drug by making it the first drug to receive clearance for use in Covid patients.
Another latest Canadian study suggests the antiviral medication Remdesivir could have a “modest but significant effect” on COVID-19 patient outcomes, including decreasing the need for mechanical ventilation by approximately 50 per cent.
While this study claims to be the extension of Solidarity trial’s arm in Canada, the disclaimers show that one of authors of the study, Conar O’Neil, is a member of a Gilead Sciences advisory board.
The American study – funded by the National Institute of Allergy and Infectious Diseases (NIAID) – named ACTT-1 showed that Remdesivir was superior to placebo (dummy pill) in shortening the time to recovery in adults who were hospitalised with Covid.
ALSO READ | EXCLUSIVE | Covid Treatment: Centre Plans Live Dashboard to Monitor Availability of Key Drugs
Here also, six authors have been linked to Gilead in some way such as receipt of grants or being employed by the company. However, disclaimer clarifies that NIAID ultimately made all decisions.
NEW PRESCRIPTION COMING SOON. READY FOR NEW EXPERIMENTS?
The data collected, so far, shows that the drug is “definitely not” recommended in mild and moderately ill patients who do not require oxygen support, nor the drug is recommended in critically ill ICU patients on ventilators or ECMO.
Arguably, some patients might go home a few days earlier assuming that the drug reduces days spent in hospitals, but we must ask if the people, time, money and resources that we invest to achieve the small benefits are really worth it?
It is a waste for the governments to invest in Remdesivir based on the idea that it might help a small subset of patients.
Now, after making many of us run for life, new studies show that this drug should be given either in home-setting or at day care (or OPD) centres of the hospitals as soon as onset of symptoms. The American regulator, US FDA has approved the drug for high-risk Covid patients who are not sick enough to be hospitalised – somewhat similar to Merck’s Molnupiravir or Pfizer’s Paxlovid. In fact, the latter two are pills whereas Remdesivir needs to be injected in veins.
Overall, the drug – which is expensive, invasive and painful – has failed to impact outcomes that matter to a patient — the reduction in death.
Another big reason to stop the use of Remdesivir is its chances to create stronger strains of coronavirus.
Former ICMR scientist Dr Raman Gangakhedkar had once told me during an interview that the administration of these antiviral treatments or plasma therapy will lead to the evolution of new drug-resistant mutations.
The indiscriminate use of treatments, which have failed to prove effective in different stages of Covid-19, “can help the SARS CoV2 virus mutate further and become stronger”, he told me.
In short, Remdesivir has hardly lived up to the hype.
We must stop abandoning scientific reasoning citing desperate times. It’s a drug searching for a disease, not vice-versa.
Read all the Latest India News here

















Comments
0 comment