views
Testing Gold with Fire
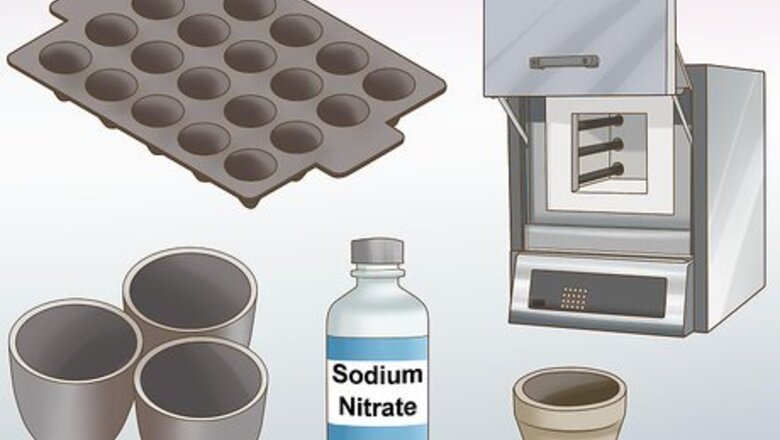
Prepare your equipment. You will need a crucible to put the sample into. You will need a heat source such as a torch or furnace to bring the sample to high temperatures. You will also need other reactants such as the additives to create the flux, the bone ash to cupel the metal, and some sodium nitrate to extract residual silver. You will also need molds to pour hot metal into. Also wear goggles, heat resistant gloves, and ideally a fireproof suit.
Place the sample in a crucible. The crucible needs to be able to withstand high temperatures. The sample will be exposed to enough heat to melt all of the metals and separate them from other minerals. Clay or ceramic crucibles can withstand tremendous heat.
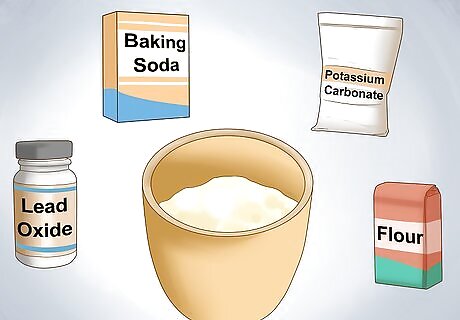
Combine any additives. Additives such as lead oxide, sodium bicarbonate, potassium carbonate, and flour are used to form a flux. The flux reacts with the same (or ore) to promote melting. Different ratios of each additive will produce slightly different flux compounds.
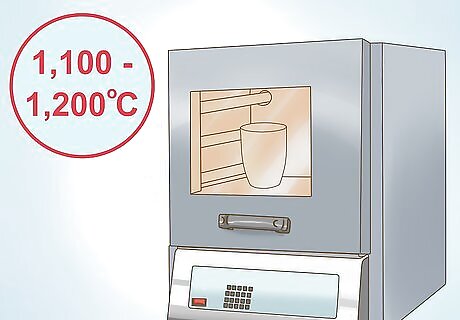
Heat the reaction to completion. The flux reaction needs to be heated to completion. When the reaction is complete, you will see two separate layers. Depending on the laboratory and the additives used, you will typically heat to between 1,100 and 1,200 degrees Celsius (2,012 - 2,192 degrees Fahrenheit). The top layer is molten glass that contains no valuable minerals. The bottom layer contains your molten precious metals.
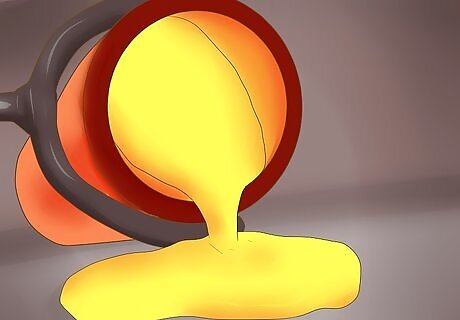
Pour off the top layer. Carefully discard the top layer of molten glass. It will be of no further use in the assay. No gold, silver, or other metal will be lost by doing this. Be careful not to pour off any of the metal layer.
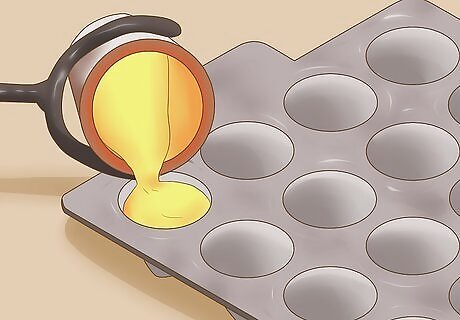
Cool the metal. Pour the metal into a mold. In the mold, the metal can cool until it once again reaches a solid state. This metal is now comprised of gold, silver, and lead. Be very careful, as the metal will be hot for a long period of time and can severely burn you.

Cupel the metal. A cupel is a porous container made of bone ash that will readily absorb lead oxide. To cupel the metal, you place it in the cupel and blast it with hot air. This will oxidize the lead. The lead oxide will then vaporize or be absorbed by the bone ash. After cupling, you will have a metal sample that is composed of gold and silver.

Dissolve the silver. Submerge the metal in nitric acid. The acid will not dissolve the gold, but it will dissolve the silver. You can then pour the solution through a filter to separate the gold.
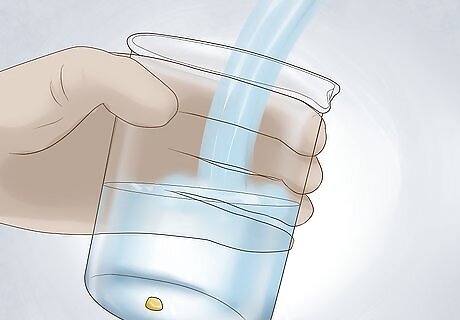
Wash the gold. Wash the gold with water to remove any excess nitric acid. Pat the gold dry with a soft towel. At this point, you should have a sample that is nearly pure gold.
Weigh the gold. With all contaminants removed, you can weigh your gold on a scale. By comparing the weight of the gold to the weight of the original sample, you can determine the percent weight of gold in your ore or scrap. This completes the fire assay of the gold piece.
Dissolving Gold in Aqua Regia
Gather the needed reagents. You will need hydrochloric and nitric acids. You will also need a filter to filter out contaminants. Finally you are going to need an oxidizing reagent. Wear goggles and gloves when working with this method.
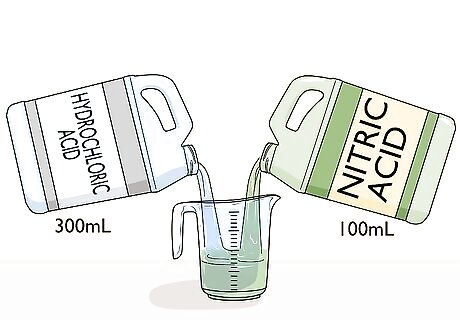
Mix acids to form aqua regia. Aqua regia is Latin for “royal water.” This solution is used to remove gold from a scrap of metal or an ore. To make it, mix three parts hydrochloric acid and one part nitric acid. For example, 400 mL of aqua regia will contain 300 mL of hydrochloric acid and 100 mL of nitric acid. Use gloves, goggles, and caution when making and using aqua regia. It is highly corrosive and toxic. Aqua regia cannot be stored well. A new batch must be made for each use.
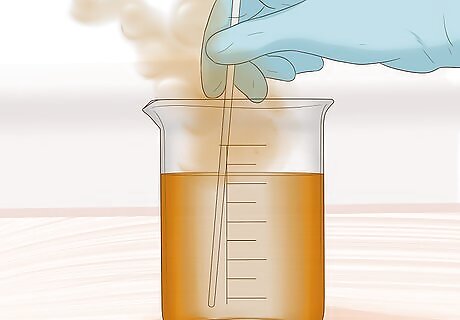
Dissolve the sample. Submerge the metal sample in aqua regia. Stir and swirl to dissolve the sample. Non-metal minerals and silver in form of silver chloride may not dissolve. These minerals will form a sludge.
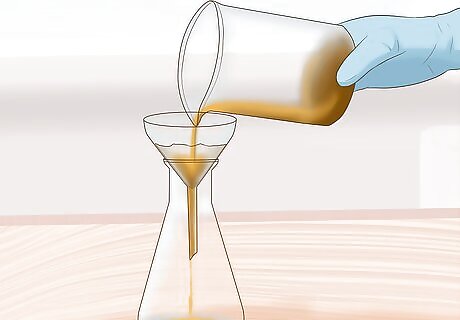
Filter the sample. Pour the sludge solution through a filter. The sludge will remain on one side of the filter and the aqua regia solution containing the metals will pass through to the other side. The solution is usually greenish in color and will contain many dissolved metals such as gold, and copper.
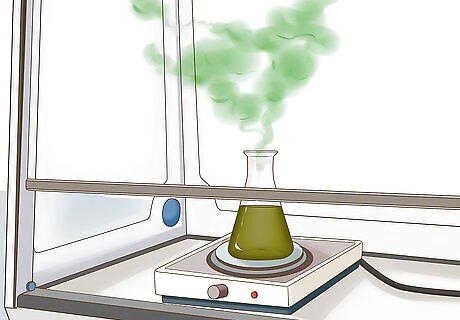
Remove nitric acid. The nitric acid must be removed before the gold can be taken out of solution. You can do this by boiling the solution. Do not breathe in the fumes. Do this outside or under a fume hood.

Precipitate the gold. The gold must be forced out of solution. To do this, you must use a reducing agent. Oxalic acid is commonly used for this. After precipitating, the gold will a solid that sinks to the bottom of the solution.
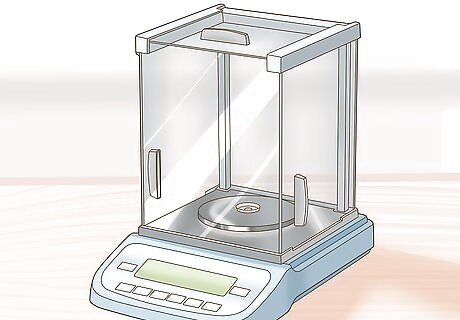
Collect and weigh the gold. Strain the gold from the aqua regia solution and dry it. Weigh the gold on a scale. The weight of the gold can be compared to the weight of the original sample to determine the ratio of gold to other metals and minerals.
Examining Gold with ED-XRF Spectrometry

Collect a sample. Samples can be collected from the field or purchased. Alternatively, any jewelry or scraps of metal can also be analyzed. The sample will not be harmed by the spectrometer
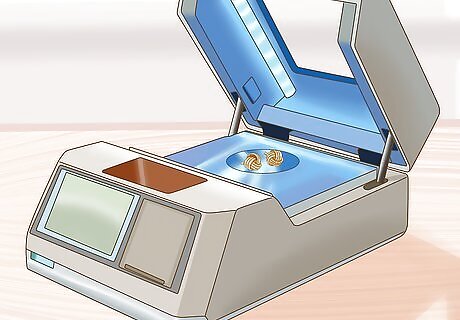
Analyze the sample. Little preparation is needed to analyze a sample with ED-XRF spectrometry. The results are extremely accurate and cost very little compared to other methods. The spectrometer can examine samples that are solid, liquid, or powdered.

Understand the results. ED-XRF spectrometry is short for Energy Dispersive - X-Ray Fluorescence Spectrometry. This technology identifies elements and compounds by the way that they disperse light. Results will show the percent composition of gold in your sample. From that, you can determine how much gold is present given the weight of the sample. For example, if you had a piece of jewelry that was 100 grams and had a 70% gold composition, the piece would contain 70 grams of gold.
















Comments
0 comment