views
Calculating Your Anion Gap

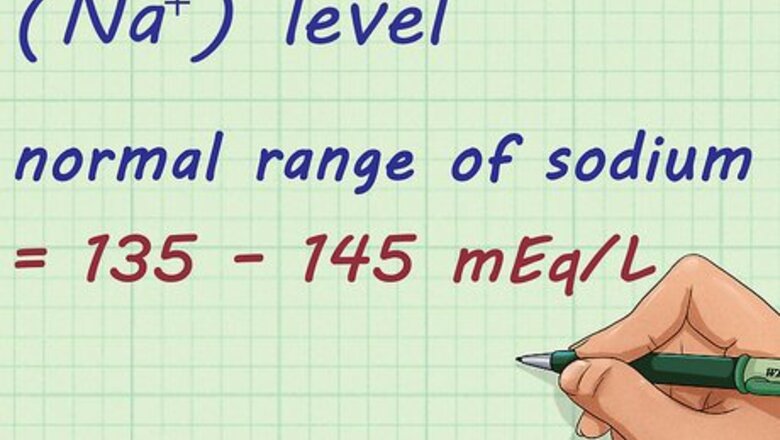
Determine your sodium (Na⁺) level. The normal range of sodium is 135 – 145 mEq/L. It is important to know the sodium level in your body. You can have your sodium levels tested through a blood test that your doctor can give.

Determine your potassium (K⁺) level if necessary. A normal range of potassium is 3.5 – 5.0 mEq/L. There’s a different formula wherein you will no longer use the potassium level, though. This is because K⁺ is found to be too low in the plasma to count at times. Since there is a formula that doesn't require potassium, you may skip this step.
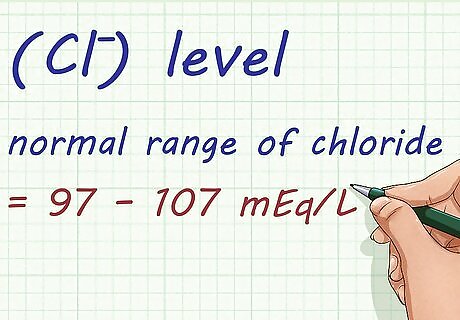
Determine your Chloride (Cl⁻) level. A normal range of chloride is between 97 – 107 mEq/L. Your doctor will test this, too.

Determine your Bicarbonate (HCO₃⁻) level. The normal range for bicarbonate is 22- 26 mEq/L. Again, this is done through the same series of tests.

Know the normal reference value of anion gap. The normal value of an anion gap is 8 – 12 mEq/L if without potassium. However, if potassium is given, the normal range value will change to 12 – 16 mEq/L. Note that all these electrolyte levels can be identified through a blood test. Pregnant people may notice different levels, too. We'll discuss this in the next section.
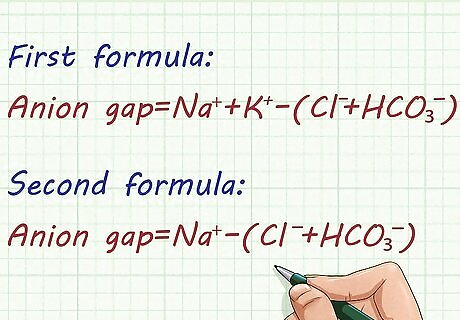
Use the given standard formula for anion gap. There are 2 formulas you can use in calculating an anion gap: First formula: Anion gap = Na⁺ + K⁺ – (Cl⁻ + HCO₃⁻). This formula can be used if there is a value for potassium. However, the second equation is used more often than the first one. Second formula: Anion gap = Na⁺ – (Cl⁻ + HCO₃⁻). You can see that potassium is omitted in this second equation. This formula is more frequently used than the other, but you can use either of the two depending on your preferences.

Know what a healthy result is. Again, the normal value is 8 – 12 mEq/L if without potassium and 12 – 16 mEq/L with potassium. Here are two examples: Example 1: Na⁺ = 140, Cl⁻= 98, HCO₃⁻= 23AG= 140 – (98 + 23)AG = 24 The anion gap is 24. Therefore, the individual is positive for having a metabolic acidosis. Example 2: Na⁺ = 135, Cl⁻= 100, HCO₃⁻= 25AG= 135 – (100 + 25)AG = 10 The anion gap is 10. Therefore, the result is normal and the person does not have metabolic acidosis. It is within the normal range of 8 – 12 mEq/L.
Understanding Anion Gap
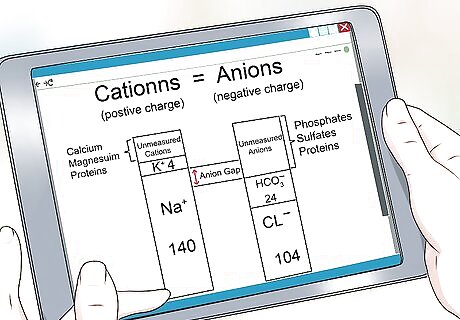
Know what anion gap really is. The anion gap (AG) measures the difference between sodium and potassium cations and the chloride and bicarbonate anions in patients who experience kidney problems and altered mental status -- in other words, your pH balance. It represents the unmeasured anion concentration in the plasma such as proteins, phosphates and sulfates. This is all fancy terminology for your body producing the right things at the wrong levels. Determining the anion gap value is crucial in setting the arterial blood gas analysis or ABG. The basic concept is that net cation and anion charges must be equal to achieve balance in your body.
Understand the significance of anion gap. This is mainly a measure for patients with kidney or gastrointestinal problems. This test does not definitely point toward any one condition. However, it does rule certain things out and narrow down the field of concern. Anion gap reveals the presence of metabolic acidosis, where the pH levels in your body are off-kilter. It differentiates the causes of metabolic acidosis and helps confirm other findings. Ask your doctor to help wrap your brain around this process. Let’s take for instance that a patient has lactic acidosis (where there's also a buildup of lactate. In this case, the serum bicarbonate levels will automatically reduce (because of the buildup) so that when you calculate for the anion gap, you’ll see that the anion gap increases.
Know what to expect during testing. A serum anion gap sample is taken from your veins using a serum separator tube. Here's how it'll go: A medical scientist or medical technologist extracts blood from a vein, likely in your arm. He or she may ask you if you have a history of latex allergies. If you do, they'll use different materials to ensure you don't have an allergic reaction. Inform them of any medical condition or medication that may cause excessive bleeding or if you have psychological issues associated with sharp items like needles. Your specimen will be kept inside a bio-refrigerator and kept in a queue for examination. When all is said and done, your doctor will contact you to discuss the findings.
Know how to interpret your results. Your doctor will correlate the diagnostic findings with how you look, feel, and the symptoms you report. Once the results are in and are definitive, your doctor should run you through what the next steps will be. If your physician thinks the results might be wrong, he may require another test to verify the results. Decreased anion gap can be correlated to various conditions such as hypo-albuminemia and bromide intoxication. A normal result is expected when a patient is recovering from diabetic ketoacidosis or recovering from bicarbonate loss due to prolonged diarrhea. An increase in anion gap may indicate lactic acidosis or renal failure. Interpretation of results may vary depending on various factors and underlying conditions experienced by the patient. A "normal" anion gap for pregnant people is slightly different. During the first trimester, normal anion gap ranges from 10 to 20 mmol/L. During the second and third trimester, normal value decreases from 10 to 11 to maximum of 18 mmol/L, respectively.
Realize that things can interfere. Collection errors can happen and interfere with your laboratory result. Timing, dilution and sample size matter a lot to get accurate findings. Delay in processing the collected specimen and exposure to air for long periods of time may cause an increase in bicarbonate levels, too. With this, anion gap may reduce by 2.5 mEq/L for every gram/dL albumin concentration deducted from the blood. Your doctor should be able to account for this (apart from avoiding it entirely). An increase in anion gap requires further testing -- including testing for serum lactic acid, drug testing, testing your creatinine levels and serum ketones -- to rule out possible causes of anion gap acidosis.



















Comments
0 comment