views
Inflating Balloons to Measure Gas Volume
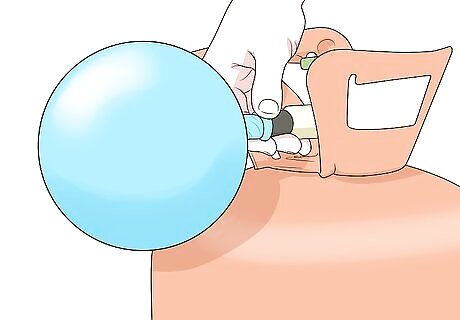
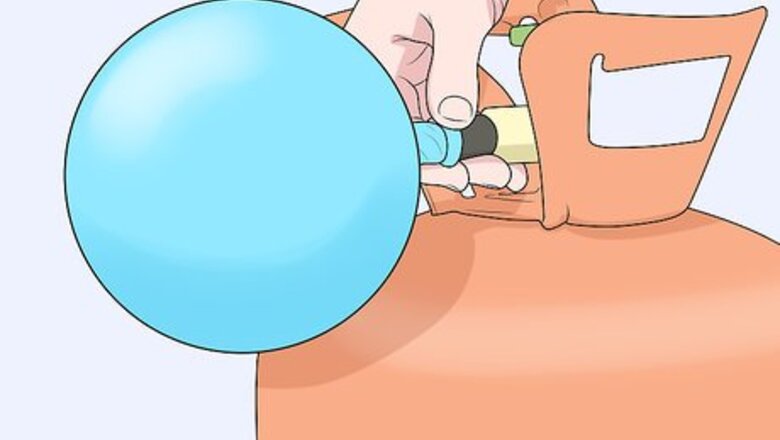
Inflate a balloon with the gas you want to measure. If you’re taking gas (like helium) from a tank, place the lip of the balloon tightly over the nozzle and fill it up. Or, if you want to measure air, just blow up the balloon using your mouth. Tie the end of the balloon in a tight knot when you’re done.
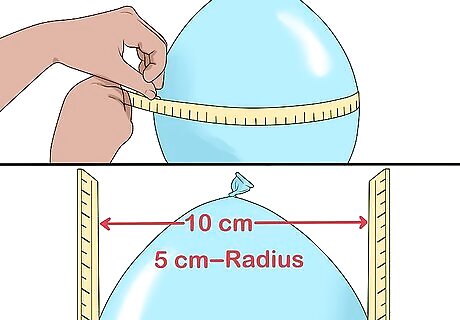
Measure the balloon's circumference and radius. Wrap a cloth tape measure around the center of the balloon to check its circumference. To get the radius of your inflated balloon, place a ruler on each side of the center of the balloon, measure the distance between them, and divide it in half. For instance, you place your balloon between two rulers and find that the distance is 10 centimetres (3.9 in). Divide this in half to get the radius, 5 centimetres (2.0 in).
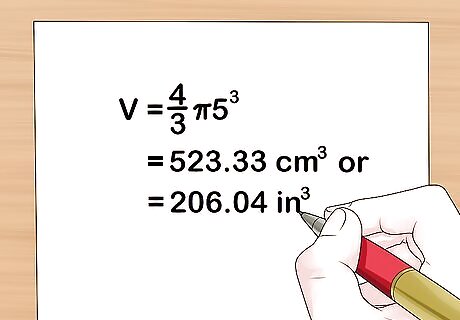
Use the volume of a sphere formula. Plug in your measurements into the basic formula for the volume (V) of a sphere, V = 4 3 π r 3 {\displaystyle {\frac {4}{3}}\pi r^{3}} {\frac {4}{3}}\pi r^{3}. Plug the radius into the formula to get your sum. For instance,: V = 4 3 π 5 3 {\displaystyle {\frac {4}{3}}\pi 5^{3}} {\frac {4}{3}}\pi 5^{3}, or about 523.33 cm 3 {\displaystyle ^{3}} ^{3} or 206.04 in 3 {\displaystyle ^{3}} ^{3}.
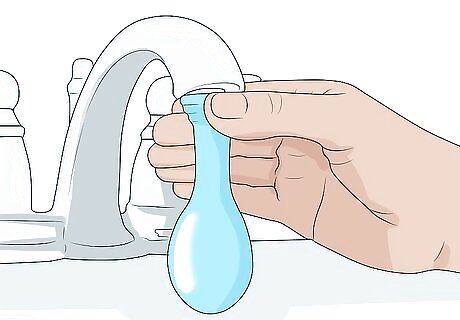
Fill up another balloon with water to compare your results. Place the lip of a balloon over a faucet and carefully run water into it. Stop when the balloon is the same size as the balloon filled with gas. If you accidentally put too much water in, carefully pour some out. It will be difficult to get the balloons to exactly the same size. To be more exact, wrap a cloth tape measure around the center of the water balloon to check its circumference. Adjust until it's the same as the gas-filled balloon's. Have someone help you measure the circumference of the water balloon while you hold it.
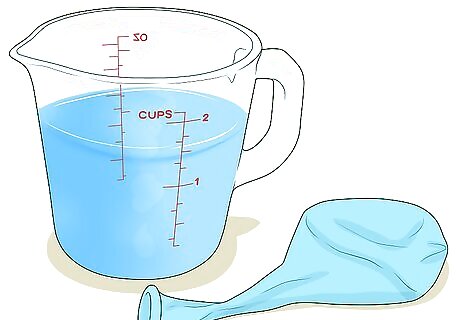
Pour the water out and measure it. Empty the balloon into a large measuring cup, beaker, or something else that can measure volume. Record the amount of water that was in the balloon. The volume of water will equal the volume of gas in the other balloon. If your water-filled balloon is the same size as the gas-filled balloon that you determined (via V = 4 3 π r 3 {\displaystyle {\frac {4}{3}}\pi r^{3}} {\frac {4}{3}}\pi r^{3}) had a volume of 523.33 cm 3 {\displaystyle ^{3}} ^{3}, then you should measure about 523 milliliters (18 fl oz) of water poured out of it (1 ml = 1 cm 3 {\displaystyle ^{3}} ^{3}).
Displacing Water to Measure Gas Volume
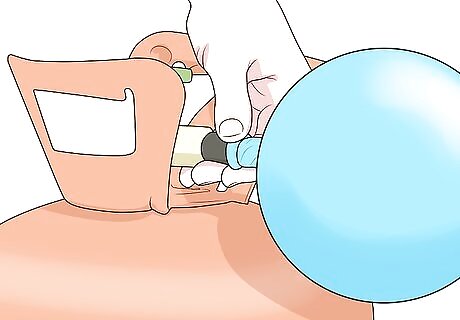
Fill a balloon with the gas. Place the end of the balloon over the nozzle on the tank of gas, and fill your balloon to size you want. If you’re measuring air, you can just blow up the balloon like you normally would. Tie the end when you’re done.
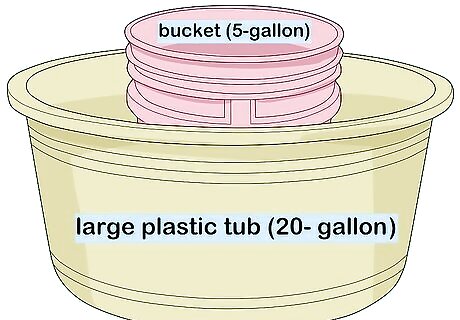
Place a smaller container inside a larger one. Find a container that’s larger than your balloon, then get another container that’s even larger than that. Place the smaller container inside the larger one. For instance, you can place a 5-gallon (about 19 liters) bucket inside a large plastic tub (20 gallons / 75 L or more).
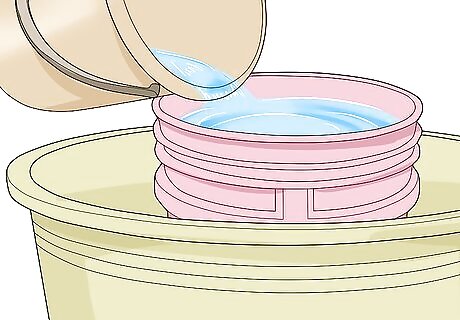
Fill the smaller container with water. Pour enough water into the smaller container so that is full exactly to the brim. Be careful not to spill any water into the larger container.
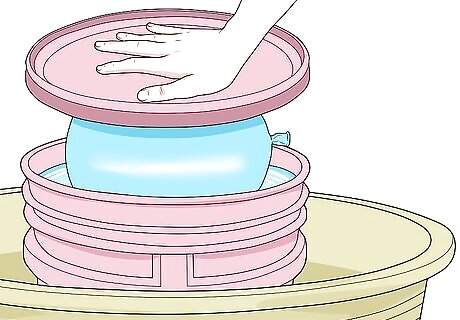
Push the balloon into the smaller container. Carefully set the balloon on top of the container of water. Push it down into the water with something flat (like the lid of a plastic tub). Keep pushing until the balloon is all the way into the water and the flat object hits the top of the bucket. Some of the water will spill into the larger container.
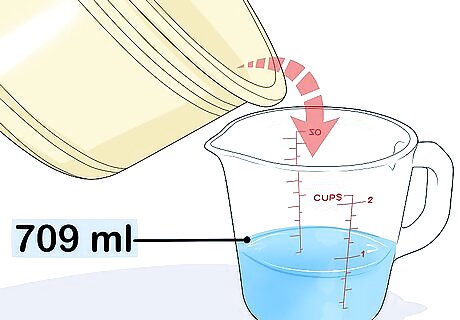
Measure the water that spilled into the larger container. Lift the smaller container of water out. Pour the water left behind in the larger container into a beaker, large measuring cup, or something similar. The volume of water will equal the volume of gas in the balloon. For example, you might find that the balloon displaced 24 ounces (about 709 mL).
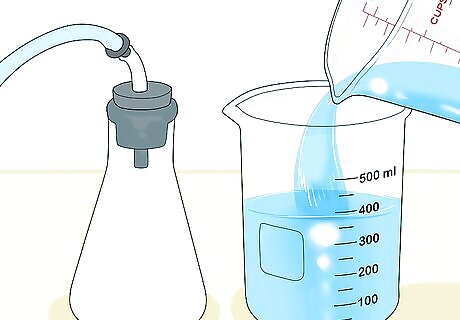
Set up a flask and beaker for a more accurate measurement. Get a flask that has a stopper with an outlet for tubing. Fill the flask with the gas you want to measure, with the stopper in place, and pinch the tubing so no gas escapes. Fill a beaker with water, place a shallow dish on top of it, and turn both upside down. Record the level of the water in the beaker using the measurement lines on its side. This technique only works for gases that are insoluble in water.
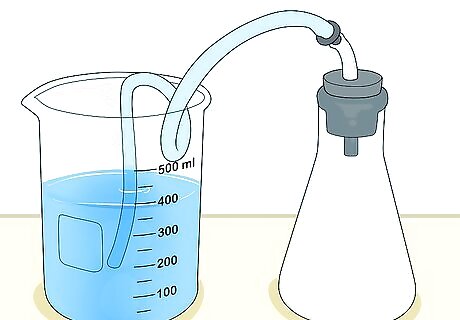
Measure the volume of the gas using the beaker. Slide the tubing underneath the edge of the beaker, in the shallow pan that has now partially filled with water. Gas will escape through the tubing and bubble up, pushing some of the water out of the beaker. Once all of the gas has bubbled up, record the water level using the measurement lines on the side of the beaker. The difference between the water levels before and after the gas entered the beaker equal the volume of the gas. For instance, if the water level was at 55 milliliters (1.9 fl oz) before the gas entered, and 65 milliliters (2.2 fl oz) afterwards (remember that the beaker is upside down, so the levels will go up numerically), then there is 10 milliliters (0.34 fl oz) of gas in the beaker.
Finding the Mass of a Gas
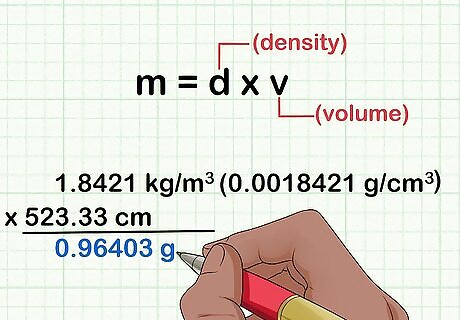
Calculate the mass of the gas, if you know its density and volume. If you know what gas you are measuring, use the formula for mass (m) using the density (d) and volume (v) of the gas: m = d × v {\displaystyle m=d\times v} m=d\times v. You'll need to look up the density of the gas in a table online or in a textbook. For instance, imagine you are measuring 523.33 cm 3 {\displaystyle ^{3}} ^{3} of carbon dioxide, CO 2 {\displaystyle _{2}} _{2}, which has a density of 1.8421 kg/m 3 {\displaystyle ^{3}} ^{3}. The mass of your sample would be 1.8421 kg/m 3 {\displaystyle ^{3}} ^{3} (0.0018421 g/cm 3 {\displaystyle ^{3}} ^{3}) x 523.33 cm 3 {\displaystyle ^{3}} ^{3}, which equals 0.96403 g.
Use a scale to find the mass instead. Put just a small piece of double-sided tape at the center of the scale and zero it. The tape will hold a balloon or other container in place. Zeroing the scale ensures that the tape won’t skew the measurement.

Weigh the container you want to fill with gas. You can weigh an empty balloon if you want, or use another container (such as a jug). Place it on the scale and record the measurement.
Fill the container with gas. Inflate the balloon or fill the container with your gas. If you’re measuring air, inflate the balloon with your mouth. Otherwise, fill your container or balloon from a tank.
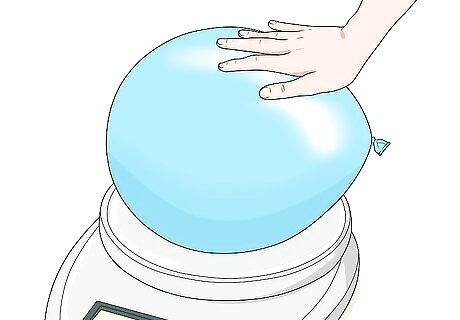
Place the inflated container on the scale. The double-sided tape will help hold the balloon or container down, if necessary. Record the measurement given by the scale. The inflated container will be greater than the empty container, but the difference may not be very much. For instance, an inflated container might weigh 8 ounces (230 g), while the empty container is 6 ounces (170 g).
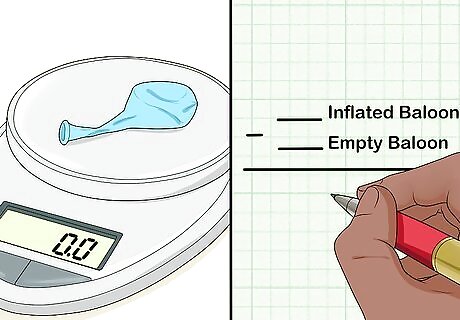
Subtract the weight of the empty container/balloon. Take the weight of the empty container away from the weight of the inflated container. This will give you the weight of the gas alone.















Comments
0 comment