views
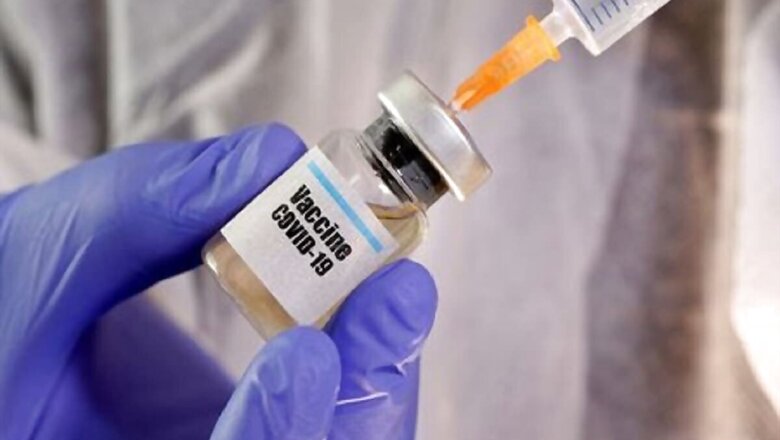
Coronavirus Vaccine Tracker LIVE Updates: The World Health Organisation on Tuesday cautioned against fast-tracking coronavirus vaccine deployment, warning that using an unproven vaccine could lead to “adverse side effects” in people and therefore, emergency use authorisations needed to be done with “a great deal of seriousness”.
The comments came just a day after the US drug regulator, the Food and Drug Administration (FDA), said it was open to grant emergency use authorisation to a coronavirus vaccine. The head of the USFDA said he would be willing to bypass the normal approval process to authorise a COVID-19 vaccine as long as officials were convinced the benefits outweigh the risks.
Opposing this route, Dr Soumya Swaminathan, chief scientist of WHO, said scientists around the world are united in a call that the approval of a vaccine must be based on data from phase-3 clinical trials. “The risk of approving a vaccine prematurely is that, first of all, it will make it very difficult to continue with randomised clinical trials. And secondly, there is a risk of introducing a vaccine that has been inadequately studied and might turn out to have a low efficacy, thereby not doing the job of bringing an end to this pandemic, or even worse, have a safety profile that is not acceptable,” she said.
“If you move too quickly to vaccinate … millions of people, you may miss certain adverse effects,” Mike Ryan, the head of the organisation’s emergencies programme, said.
AstraZeneca’s COVID-19 vaccine candidate begins late-stage US study
AstraZeneca Plc said on Monday it has begun enrolling adults for a U.S.-funded, 30,000-subject late-stage study of its high profile COVID-19 vaccine candidate. Trial participants will receive either two doses of the experimental vaccine, dubbed AZD1222, four weeks apart, or a placebo, the company said.
The trial is being conducted under U.S. government’s Operation Warp Speed program, which aims to accelerate development, manufacturing and distribution of vaccines and treatments for COVID-19. US President Donald Trump has said a vaccine for the novel coronavirus could be available before the Nov. 3 presidential election, much sooner than most experts anticipate.
AstraZeneca, which is developing its vaccine in conjunction with Oxford University researchers, and Pfizer Inc with partner BioNTech SE have said they could have data by October to support U.S. emergency use authorization or approval of their respective vaccines. AZD1222 is already undergoing late-stage clinical trials in Britain, Brazil and South Africa, with additional trials planned in Japan and Russia. The trials, together with the U.S. Phase III study, aim to enroll up to 50,000 participants globally.
Brazil’s Bolsonaro says nobody will be forced to have vaccine
Brazilian President Jair Bolsonaro, who has consistently downplayed the severity of the coronavirus outbreak, said on Monday that nobody will be forced to have the vaccine against the pandemic once it is developed. The comments come after the government earmarked millions of dollars for the purchase and future production of vaccinations as Brazil suffers the second worst outbreak of the pandemic outside the United States.
“No one can force anyone to get a vaccine,” he said in response to a question from a supporter, according to a video posted on social media. Brazil has become a hot spot in recent months, with 3,908,272 confirmed cases and 121,381 deaths from COVID-19, the disease caused by the virus.
Russia to vaccinate high risk groups towards end of year: agencies
Russian Health Minister Mikhail Murashko has said that mass vaccination of high risk groups in the country against COVID-19 would begin in November-December this year, Russian news agencies reported. Russia this month became the first country to grant regulatory approval to a COVID-19 vaccine after less than two months of human testing, prompting international experts to question its safety and efficacy.
EU offers 400 million euros to WHO-led COVID-19 vaccine initiative
The European Commission said on Monday that it would contribute to an initiative led by the World Health Organization to buy COVID-19 vaccines, while the WHO said Germany had joined the pact and that the agency was still negotiating with the bloc.
The Commission, announcing that it would provide 400 million euros ($478 million) in guarantees, did not clarify whether EU states would acquire shots through the WHO scheme. “Germany has joined the COVAX facility today,” WHO director-general Tedros Adhanom Gheybreyesus told a news conference in Geneva without elaborating the terms.
Scientists see downsides to top COVID-19 vaccines from Russia, China
High-profile COVID-19 vaccines developed in Russia and China share a potential shortcoming: They are based on a common cold virus that many people have been exposed to, potentially limiting their effectiveness, some experts say.
CanSino Biologics’ vaccine, approved for military use in China, is a modified form of adenovirus type 5, or Ad5. The company is in talks to get emergency approval in several countries before completing large-scale trials, the Wall Street Journal reported last week. A vaccine developed by Moscow’s Gamaleya Institute, approved in Russia earlier this month despite limited testing, is based on Ad5 and a second less common adenovirus.


















Comments
0 comment