views
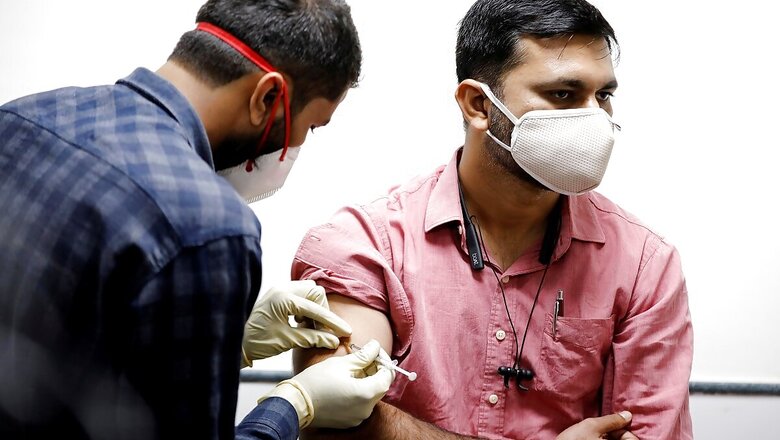
The Serum Institute of India, which is manufacturing the Oxford-AstraZeneca vaccine, has already stockpiled 40-50 million dosages of the Covid-19 vaccine ahead of the inoculation drive in India, the company’s CEO Adar Poonawalla said on Monday, adding that the firm will be able to produce around 300 million doses by July next year.
The emergency use authorisation for the Oxford-AstraZeneca vaccine is expected within days and four states – Punjab, Gujarat, Assam and Andhra Pradesh – on Monday began a trial run of vaccine delivery systems, checking everything from their technology platforms to the storage infrastructure that will be required to inoculate millions.
“Once we get regulatory approvals in a few days, it’ll be down to the government to decide how much they can take and how fast,” Poonawalla said. India wants to deliver 600 million coronavirus shots in the next six to eight months starting in January.
Along with the Oxford vaccine, the country’s drug regulator is also considering similar approvals for the Pfizer/BioNTech vaccine and another developed by Hyderabad-based Bharat Biotech.
Government sources had last week said that the Oxford-AstraZeneca vaccine is likely to get regulatory approval for emergency use in India by the New Year as the updated data submitted by Serum Institute of India (SII) appears “satisfactory”. It scores over its competitors on two critical counts – ease of storage and cost.
The Indian drug regulator is looking at the UK, which sources believe may give its nod to the Oxford vaccine before the New Year as well, before deciding on giving emergency use authorisation to the Serum Institute.
If approved, the Oxford-AstraZeneca shot will be the second vaccine available in the UK after the one made by Pfizer-BioNTech, which was approved by British regulators earlier this month but is more expensive and requires extremely low temperatures for safe storage.
Speaking to reporters, Poonawalla said the first six months of 2021 will see a shortage of vaccines globally, but said he expects an easing off by August-September 2021 as other vaccine manufactures also will be able to supply by then.
He said all the data on the Oxford vaccine has been submitted in India and UK and no concerns have been found. “You will be hearing some good news from the UK very soon,” Poonawalla told reporters, adding that approval from the Indian regulator would likely follow shortly. “By January, we should have the AstraZeneca/Oxford vaccine licensed.”
Last month, AstraZeneca had released phase three trial data for its vaccine candidate and found that it was between 62 per cent and 90 per cent effective in protecting against Covid-19 in different dosages.
But on Sunday, Pascal Soriot, the chief executive of AstraZeneca, told British daily The Sunday Times that the Oxford coronavirus vaccine will “protect 95 per cent of patients” and is “as effective as the Pfizer and Moderna” alternatives, adding scientists had figured out a “winning formula to get efficacy up there with everybody else”.
Read all the Latest News, Breaking News and Coronavirus News here













Comments
0 comment