views
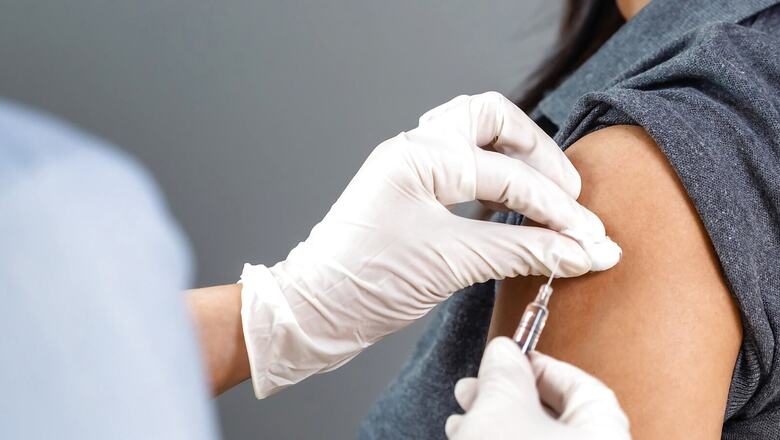
Global healthcare major Johnson & Johnson on Saturday received Emergency Use Authorization (EUA) for its single-dose COVID-19 vaccine in India, Union Health Minister Mansukh Mandaviya tweeted. The company had revealed it has sought EUA in India on Friday.
How is Johnson & Johnson vaccine different from its counterparts like Moderna and Pfizer?
Unlike its US counterparts, Moderna and Pfizer vaccines, the Johnson & Johnson vaccine does not use messenger RNA (mRNA) to help the body build its defenses against the virus. Instead, it is an adenovector vaccine like the Oxford-AstraZeneca vaccine. In this case, the gene of the coronavirus’ signature spike protein is added to an adenovirus, a common virus that causes colds or flu-like symptoms, which when introduced into the body, delivers the instructions that teach human cells to make the spike protein. That causes the immune system to react by making antibodies to attack the spike protein.
How did the J&J vaccine have the first mover’s advantage?
A history of the vaccine shows that its central formula was in making over a decade before the outbreak of the coronavirus . Dan Barouch, a virologist at the Beth Israel Deaconess Medical Center, and his team had been developing a “vector” for the last decade and a half, as per which a part of a pathogen’s genetic code would be introduced into human cells. Once there, it would trigger the cells to create pieces of the pathogen for the body’s immune system to identify and attack.
What is the efficacy of the Johnson & Johnson vaccine?
Johnson & Johnson vaccine is currently the only vaccine, which is effective after only one dose. Authorities can use this vaccine on people who may be hard to reach or who are otherwise unlikely to get a second dose.
Unlike all Indian vaccines, which have to be stored at 2-8 degrees Celsius, the
Johnson & Johnson vaccine can be refrigerated for up to three months at normal temperatures. This would allow the government to send these stocks to rural parts of the country where the cold chain may not be well developed.
Johnson & Johnson said a study showed the vaccine was 85 percent effective against “severe/critical disease and demonstrated protection against hospitalisation and death”.
What is the recommended dosage?
According to the Strategic Advisory Group of Experts of WHO, the single dose (0.5 ml) Janssen Ad26.CoV2.S vaccine should be administered intramuscularly. Further, there should be an interval of 14 days before the administration of this vaccine and any other vaccine against other health conditions.
But what are controversies around the vaccine?
The Johnson & Johnson vaccine has been plagued by one controversy after another. For instance, On 13 April, the US government paused the administration of the vaccine to investigate a few cases in which people experienced blood clots after receiving the vaccine. All of the cases emerged within two weeks of vaccination. But on 23 April, US health officials lifted the pause after scientific advisers decided the vaccine’s benefits outweigh the risks.
Again, earlier in the month social media users shared posts, which claimed that the vaccine contains aborted fetal DNA as an ingredient, to which the Roman Catholic Archdiocese of New Orleans released a statement calling it “morally compromised”. It was later clarified that while the vaccine used lab-replicated fetal cells during its production process, the vaccine itself does not contain any fetal cells.
Read all the Latest News, Breaking News and Coronavirus News here.


















Comments
0 comment