views
It may take some more weeks for anti-viral drug remdesivir to available to COVID-19 patients in India as pharmaceutical companies, seeking to manufacture them domestically, are still working on furnishing safety and quality data, even though the approval process for its introduction is being fast-tracked.
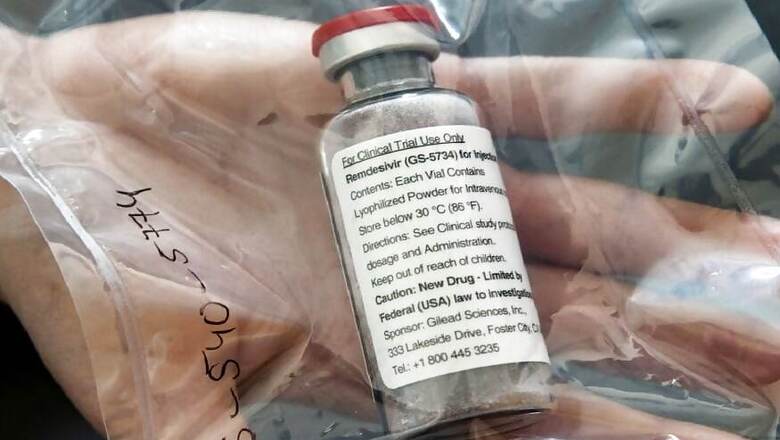
India's drug regulator had on June 1 granted US pharma giant Gilead Sciences marketing authorisation for its drug remdesivir, being touted as a potential treatment for COVID-19, in the country.
Four companies -- Hetero, Jubilant Life Sciences, Cipla and Mylan NV -- with which the original drug maker, Gilead Sciences Inc, have entered into non-exclusive licensing agreements are still awaiting nod from the DCGI for manufacturing and selling of remdesivir in India.
The Union Health Ministry on Saturday, in its revised 'Clinical Management Protocol for COVID-19', recommended the use of remdesivir under emergency use authorization on patients with moderate disease -- that is those on oxygen support. It is not recommended for those with severe renal impairment and in children less than 12 years, pregnant and lactating women.
"Four pharma companies which have applied for permission to manufacture and sell remdesivir in India are yet to furnish the test report and first-point stability data.
"Some of them have produced batches of the drug. Testing its molecular compound will be done at our CDSCO lab to check on the quality and safety aspects. All possible efforts are being made to make the drug available in the market at the earliest," a source in the know of the developments told PTI.
The approval process for remdesivir was accelerated in view of the emergency situation and the unmet need for medicines in light of the coronavirus outbreak.
"The drug has been allowed for restricted emergency use for a maximum five-day period for treatment of suspected or laboratory-confirmed cases of COVID-19 in adults and children hospitalised with severe symptoms, subject to several safeguards.
"The drug, which is administered in the form of an injection, has been approved to be sold by retail on the prescription of specialists for use in hospital or institutional set-up only," they said.
The approval process for remdesivir was accelerated by invoking special provisions under the New Drug and Clinical Trial Rules, 2019, which provides for waiver of clinical trials in special circumstances.
Gilead Sciences, the patent holder of the drug, has the complete data about the pre-clinical and clinical studies for remdesivir. The drug has been issued an Emergency Use Authorization (EUA) by the United States Food and Drug Administration (FDA) for the treatment of hospitalized patients with severe COVID-19.


















Comments
0 comment