views
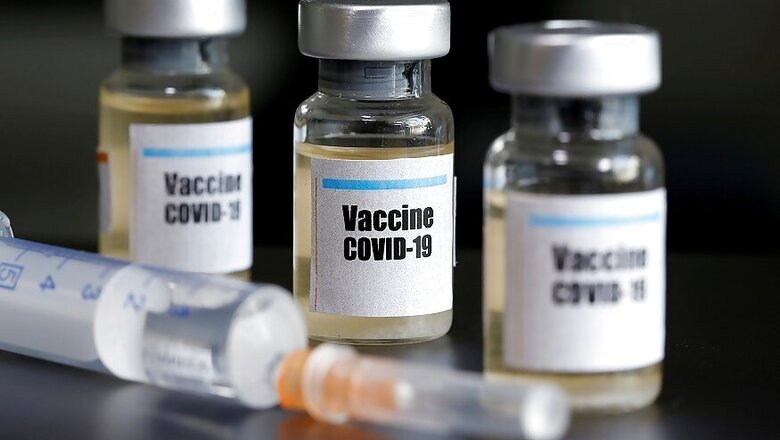
A prominent British laboratory is forming a special partnership that would sidestep the drug industry to sell a potential vaccine against the coronavirus without profits or licensing fees in Britain and in low- and middle-income countries.
Scientists, nonprofit groups and public health experts have urged that any successful vaccine to combat the pandemic be distributed at the lowest possible cost and on the basis of need rather than profit. But for-profit drug giants or biotechnology startups have dominated the development race, especially in the United States, a vital market because of its high drug prices.
The British laboratory, at Imperial College London, could alter that landscape, in part because its technology has the potential to develop a vaccine that is cheaper and easier to manufacture than others, said Robin Shattock, the lead scientist on the project.
If successful, he said, the vaccine’s lower cost could appeal to the large donor organizations that typically supply low-income countries, which make up much of the world. It could also provide a cheaper alternative in affluent countries.
“Somebody who’s developing a product that’s going to be of very high cost will actually ultimately lose out if the high-volume market doesn’t support that,” Shattock said.
Clinical trials are beginning this month, and if the vaccine is proven safe and effective, the first doses could be available early next year.
To make the vaccine as widely and cheaply available as possible, Shattock said, Imperial College is creating what it calls a “social enterprise” — a special-purpose, for-profit company chartered to sell the inoculation.
Imperial College is forming the company in partnership with the investment firm Morningside Ventures, which is based in Hong Kong. The new entity will be called VacEquity Global Health.
Morningside Ventures was founded by the Chan family, which is also a major donor to the T.H. Chan School of Public Health at Harvard University.
Imperial College has promised that VacEquity Global Health will make its vaccine available at the lowest possible cost in Britain as well as in low- and middle-income countries. VacEquity would work with specialized drug manufacturers in a process similar to the production and sale of generic drugs.
The new company may charge higher prices in wealthier countries such as the United States, Singapore or the Persian Gulf monarchies.
A clinical trial involving 300 participants in Britain — an unusual combined phase one and two — is set to begin June 15. If that shows the drug to be safe, Imperial College will conduct a 6,000-participant phase in October to test the vaccine’s effectiveness. The location of the later phase will depend on where the virus is spreading rapidly at the time.
The Imperial College is using a novel technology that has never before produced a licensed vaccine. It is described as self-amplifying RNA and was pioneered by Shattock over decades of research. The vaccine consists of specially engineered genetic material — RNA — that instructs muscle cells in the body to produce a distinctive “spike” protein found on the surface of the coronavirus.
If the vaccine is successful, those proteins will trigger an immune response that will kill the virus.
Moderna, a biotechnology company based in Cambridge, Massachusetts, has started clinical trials for a vaccine using similar technology, known as messenger RNA. The U.S. government has agreed to provide as much as $483 million to Moderna to advance its research, and reports of positive results in the trial’s early stages have sent the company’s stock soaring.
Imperial College’s self-amplifying RNA vaccine, Shattock said, would require a much smaller dose than the Moderna vaccine — 50 to 100 times smaller — which would greatly lower the cost per dose. The Imperial College vaccine would also require smaller and less costly manufacturing facilities than vaccines using other technologies, such as those involving the neutralized or modified versions of existing viruses, he said.
The British government has provided more than $50 million in financial support for the Imperial College effort, and it has also attracted $5 million from other donors.
The University of Oxford, which is beginning phase three clinical trials of an alternate potential vaccine, has tried a different approach to low-cost distribution. The university reached an unusual agreement with British pharmaceutical giant AstraZeneca, which has pledged to distribute the potential vaccine at no profit for the duration of the pandemic.
AstraZeneca has already received hundreds of millions of dollars from the U.S. government, the British government and major nonprofit organizations to begin manufacturing as many as 2 billion doses of Oxford’s potential vaccine even before its effectiveness has been proven.
If demand persists after the pandemic has faded — perhaps persisting as a seasonal virus — AstraZeneca has said it may seek to profit from the sale of the vaccine.
David D. Kirkpatrick c.2020 The New York Times Company


















Comments
0 comment