views
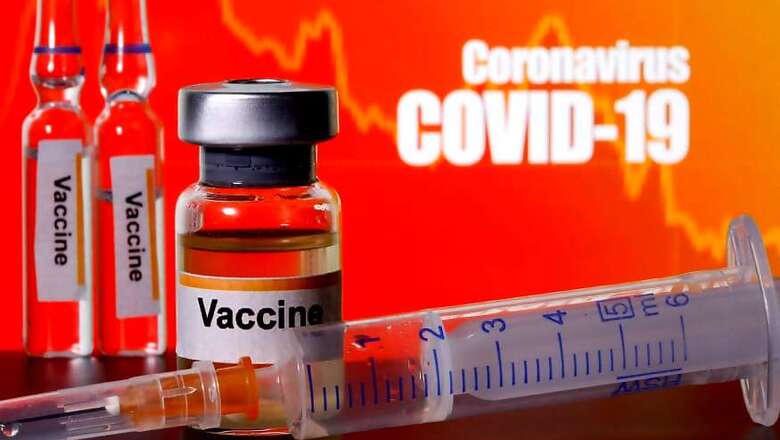
New Delhi: Bharat Biotech on Tuesday announced recruitment of 13,000 volunteers and continued progress towards achieving the goal of 26,000 participants for the Phase III clinical trials of its COVID-19 vaccine 'Covaxin' across multiple sites in India. Covaxin has been evaluated in approximately 1,000 subjects in Phase I and Phase II clinical trials, with promising safety and immunogenicity results, Bharat Biotech said in a statement.
The indigenously developed COVID-19 vaccine has been developed in collaboration with the Indian Council of Medical Research (ICMR) – National Institute of Virology (NIV), it added. "Covaxin is a highly purified and inactivated 2 dose SARS-CoV2 vaccine, manufactured in a vero cell manufacturing platform with an excellent safety track record of more than 300 million doses," Bharat Biotech said.
According to the company, the vaccine is developed and manufactured at Bharat Biotech's BSL-3, biocontainment facility. The company has successfully recruited 13,000 volunteers for the Phase III clinical trials of the vaccine and the target is to have 26,000 participants, as per the statement.
"This is an unprecedented vaccine trial ever to take place in India, and we are overwhelmed with the steady rise in participation,"Bharat Biotech Joint MDSuchitra Ella said. The company had started the phase III clinical trials in mid-November.
.
Read all the Latest News, Breaking News and Coronavirus News here















Comments
0 comment