views
Making a Saturated Solution
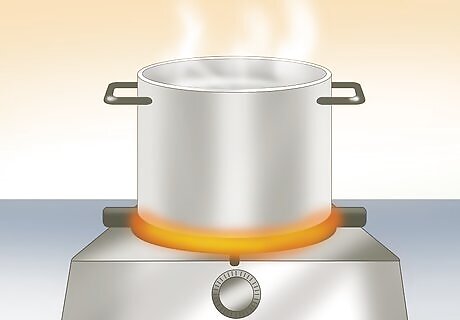
Heat some water. You can heat the water on the stove, but do not bring it to a boil. You can also simplify this step by using hot tap water. If your tap water does not get very hot, you can microwave the water for 45 seconds. The water should be almost boiling. Do not boil water without adult supervision. Try the experiment with 1 cup (240 mL) of water.
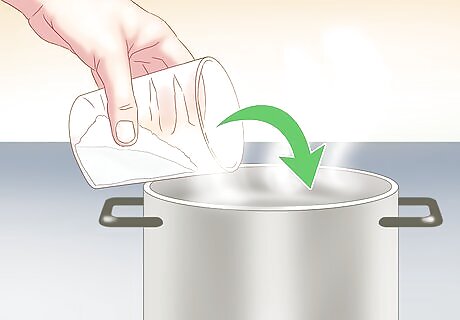
Add Epsom salt to the water. In a big bowl, add the Epsom salt in a 1:1 ratio to the water. This means that for 1 cup (240 mL) of water, you will add 1 cup (240 mL) of Epsom salt. This will allow the solution to become fully saturated. You will notice that a small amount of Epsom salt remains in the bottom of the container undissolved. Epsom salts will not react with traditional pots or stir sticks in any meaningful way.
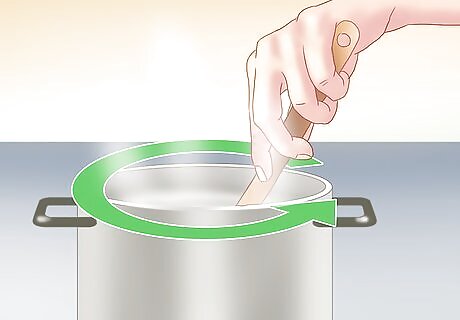
Stir the solution. You should stir the solution for about two minutes. This will allow as much salt as possible to dissolve. If you dissolve too little salt, the crystals will not form correctly.
Growing the Crystals
Chill the solution. Place the solution in the freezer for ten minutes. This allows the temperature of the solution to drop rapidly for the first few minutes. This will prepare the solution to go into the refrigerator and will typically yield better crystals.

Leave the solution overnight. Place the solution in the refrigerator and leave it overnight. Two things will happen. First, the decreased humidity in the refrigerator will allow some of the water to evaporate from the container. Second, the cooler temperature will force the water molecules to contract and get closer together. This makes less space in the solution for salt molecules and forces them to come together as a solid (crystals). You can leave the jar covered or uncovered while sitting. Just be sure not to tip it over.
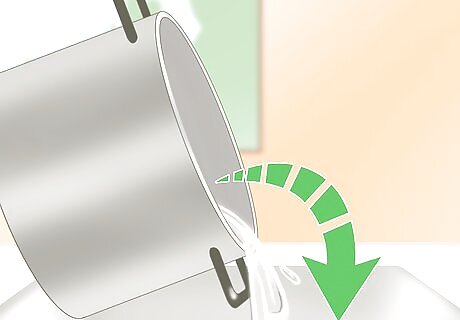
Pour off the excess liquid. When you remove the solution the next day, pour off the excess liquid immediately. Be careful not to upset any crystals that have formed. If you allow the crystals to remain in the water, they will redissolve as the water heats up.
Observing and Tweaking Results
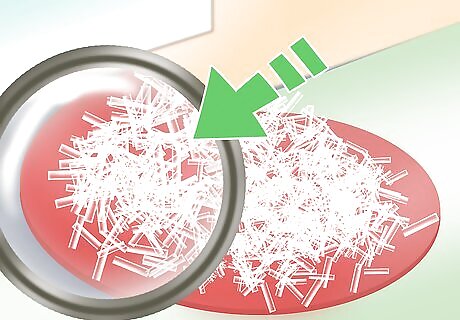
Observe the crystals. Once you have poured off the excess liquid, you can look at your crystals. If you do the experiment more than once, or in multiple containers, you’ll notice that all of the crystals have a similar shape and structure. That’s because the same chemicals (in this case magnesium and sulfate) will always form the same kind of crystal.
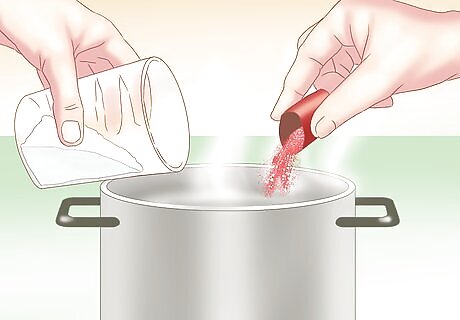
Try it with food coloring. Epsom salt forms a white crystal. If you want colorful crystals to observe, try adding food coloring to the solution. You can also add watercolor paints for a splash of color.
Leave the solution longer. Overnight crystals are a great way to get kids started with a science experiment. If you want to take it a step further, leave the crystals in the refrigerator for several days, or longer. This will allow them more time to grow and result in larger, more developed crystals.















Comments
0 comment