views
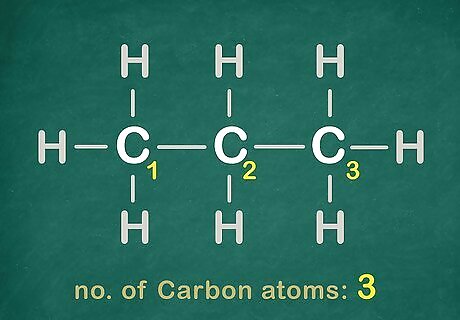
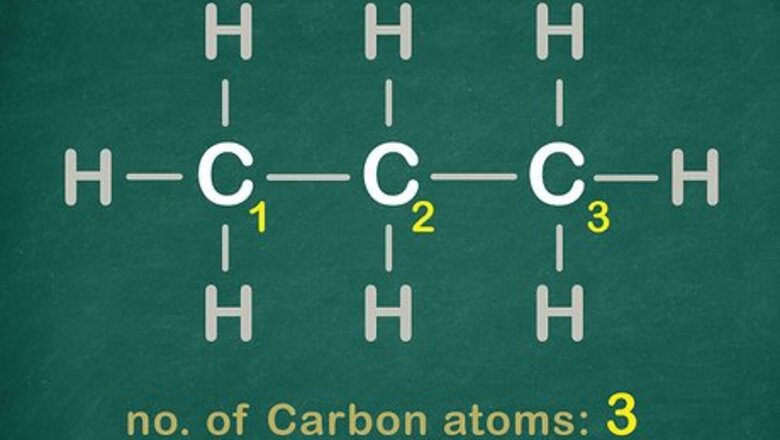
See how many Carbon atoms are in the chain.

Look at the table to determine what its name is. For 'A level' you must memorize all of names in this table.
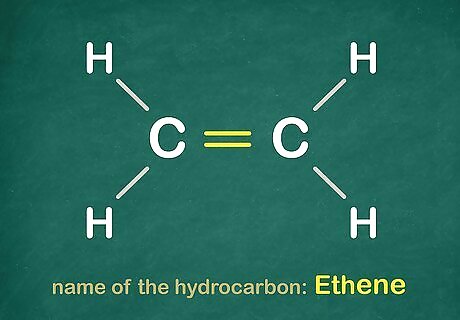
Note the other variant. The other variation of the hydrocarbon is to have a double bond in them, like this. Replace the -ane with an -ene. For example this would be Ethene.
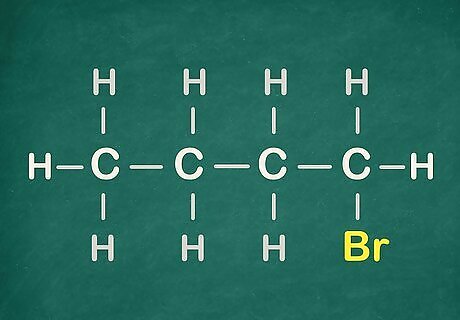
Examine the Haloalkane! The halo- in its name comes from Halogen. Learn how to name them from reading the next steps.
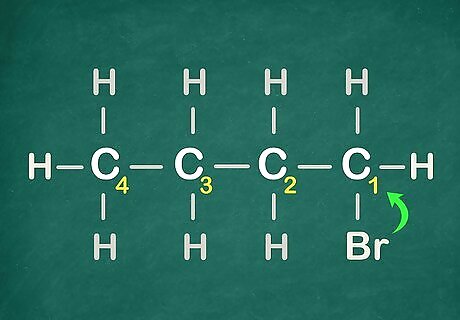
Number the carbons, starting from the Halogen (or functional group).
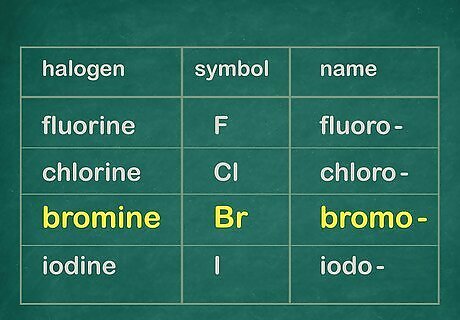
Use this table. You should find the name you will add to your chain's name.
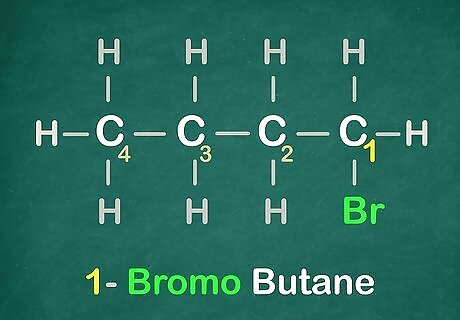
Name it. Keep in mind that the "-" is essential for naming compounds. It must always be between a number and a letter, like this "2-chloro...."
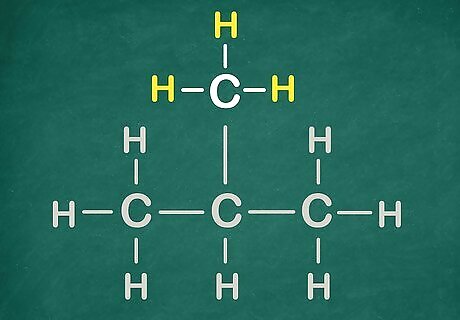
Know what a functional group! This is a functional group.
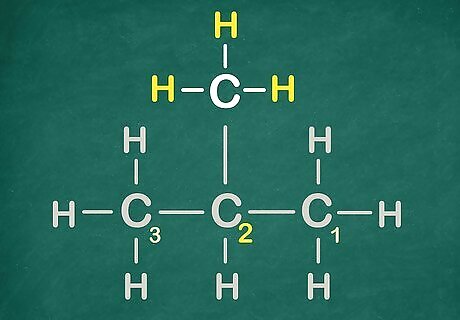
Name a function group. To do so, make sure to count from the side that gives it the smallest number. In this case it can only be 2.

Use this table to name it. It is laid out like this.Name Organic Compounds (Simple) Step 11 Version 2.jpg
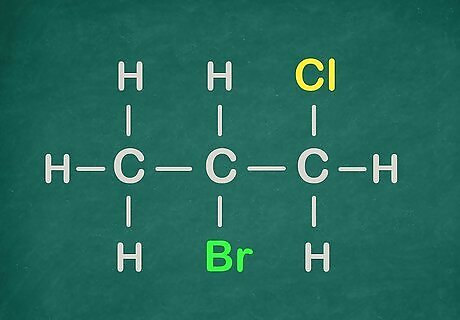
Introduce two Halogens into the hydrocarbon.
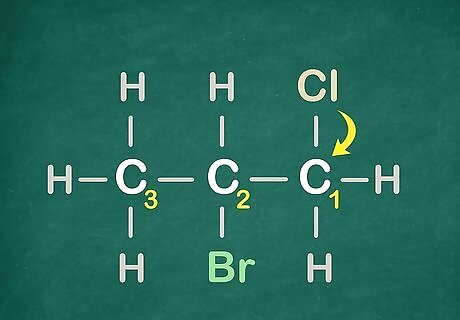
Count out on which number carbon the Halogens are attached to. Remember to keep the lowest numbered carbons.
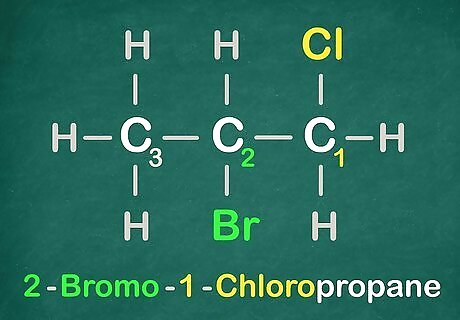
Remember another essential piece of information.
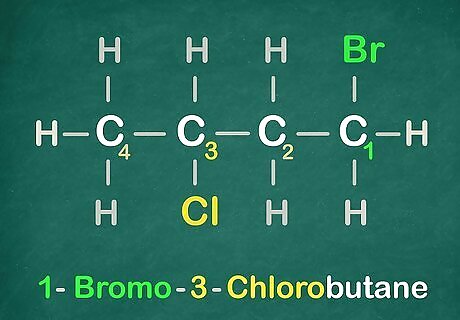
Get the Halogens in alphabetical order. Example: Bromo comes before Chloro. Use this information here is for the name of this molecule.



















Comments
0 comment