views
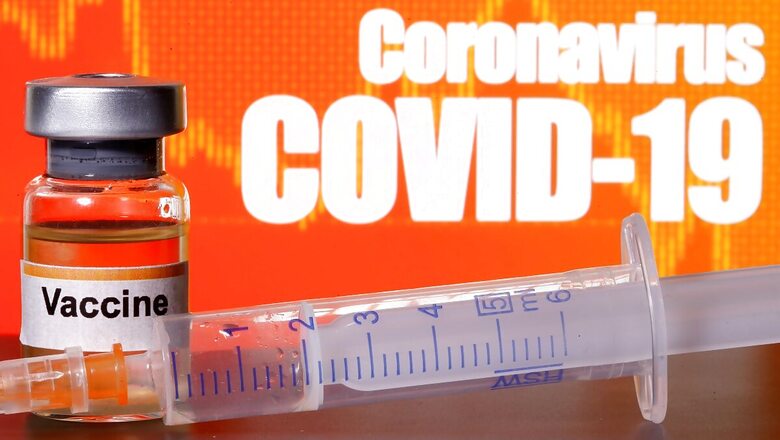
Union Minister of Health and Family Welfare Harsh Vardhan said on Saturday the country will have a vaccine against the deadly coronavirus by the end of the year.
Speaking to reporters, he said a Covid-19 vaccine is likely in the next 4-5 months.
"I hope that if everything goes well, India will have access to a coronavirus vaccine by the end of 2020," he later tweeted.
The Ministry of Health and Family Welfare said one of the three Covid-19 vaccine candidates has entered the third phase of the pre-clinical human trial.
According to VK Paul, head of the national task force on Covid-19, the vaccine candidate entering the third phase has yielded encouraging results in the initial phases of its trial. He said the other two vaccines are currently in Phase-I or II of their pre-clinical trials.
However, they did not reveal the names of the vaccines while sharing the status of their testing phase.
While the officials did not reveal the names of the vaccine candidates while speaking about their testing phase, it could be gathered that the vaccine entering the third phase is Bharat Biotech's Covaxin, jointly developed with the Indian Council of Medical Research (ICMR).
India currently has three vaccine candidates for Covid-19 -- ChAdOx1, developed by Oxford University and manufactured jointly by the Serum Institute of India (SII), Pune, and AstraZeneca; Bharat Biotech's Covaxin, jointly developed with the Indian Council of Medical Research; and the third is ZycovD by Zydus Cadila.
The SII had earlier said of starting the Phase 2 trial of its Covid-19 vaccine this week. It added it has shortlisted 10 centres across India to host Phase 2 and 3 human clinical trials.
Meanwhile, Zydus Cadila administered the second dose of its Covid-19 vaccine a few days ago.
The developments on the vaccine came a day after the National Expert Group on Vaccine Administration met five domestic vaccine manufacturers to review the clinical trial stages of these candidates. The manufacturers included two whose products are not yet in the clinical trial stage in India.
Zydus Cadila had stated in the review meeting that it may be able to launch the vaccine by next year.
















Comments
0 comment