views
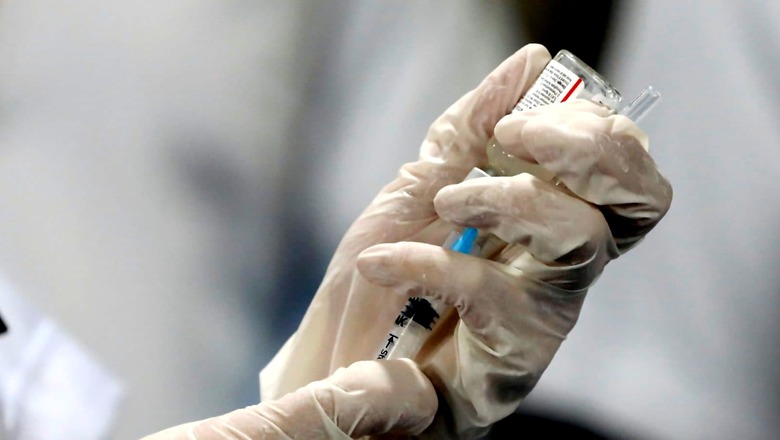
The Subject Expert Committee (SEC) of India’s drug authority is set to meet to examine Bharat Biotech’s application for its intranasal Covid vaccine as a booster or a third dose, CNN-News18 has learnt. The firm has proposed to use this vaccine as a booster dose in people already inoculated with Covaxin and Covishield.
The meeting of the SEC of the Drug Controller General of India (DCGI) is scheduled to take place at noon on Tuesday, sources say.
In its application, the company has proposed to use its intranasal vaccine BBV154 as a heterologous booster dose in already vaccinated individuals, who have received two doses of either Bharat Biotech’s own Covaxin or Serum Institute’s Covishield.
Bharat Biotech aims to conduct clinical trials on 5,000 healthy subjects: half or 2,500 individuals who have received Covishield and another 2,500 who have been administered Covaxin. The interval between the second and the intranasal booster dose is expected to be six months.
In mid-December 2021, the company sought permission to conduct a late-stage trial for a booster dose of its intranasal Covid-19 vaccine from the drug regulator.
This is a needle-free, non-invasive and easy to administer vaccine, which is expected to be suitable for both children and adults.
India is yet to approve the use of Bharat Biotech’s ‘game changer’ nasal vaccine.
Prime Minister Narendra Modi in his December 25 address to the nation had assured that soon the country will develop a nasal vaccine and the world’s first DNA vaccine.
Read all the Latest India News here
















Comments
0 comment