views
The country’s first vaccine for dengue is now getting a step closer, as the Indian Council of Medical Research (ICMR) is planning to submit the clinical trial protocol to the nation’s drug regulator by June, News18.com has learnt.
The phase 3 trials of the vaccine are likely to begin by October.
The World Health Organization (WHO) identified dengue as one of the top ten global health threats in 2019. As of now, there is no specific treatment for dengue or severe dengue.
Painful and deadly
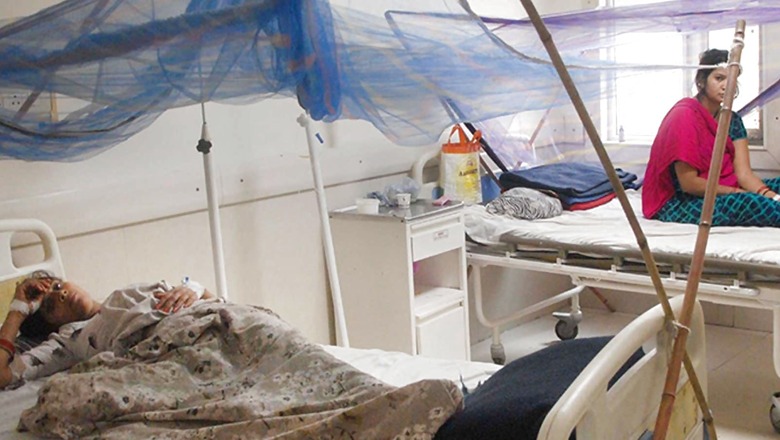
The viral disease is borne by mosquitoes, mainly of the Aedes aegypti variety. Symptoms can include fever, nausea, vomiting, aches, and muscle, joint, or bone pain so excruciating that the disease is also known as ‘breakbone fever’. If the infection progresses to severe dengue, affected people may experience shock, internal bleeding and organ failure, and it may even lead to death.
The ICMR, in May, will complete the process of signing the agreement with two pharmaceutical or vaccine-making companies – Panacea Biotec and Serum Institute of India.
“The department is working on the trial protocol and once final, it will be submitted to the Central Drugs Standard Control Organisation (CDSCO) for clearance. The anticipated timeline for the submission is six to eight weeks from now,” Dr Nivedita Gupta, the head of virology at ICMR, told News18.com. “There is an urgent need to develop effective vaccines against dengue viral disease.”
A trial protocol is a detailed plan on how the clinical trial will be conducted. It includes information such as the number of participants, number of trial locations, dosage of vaccine, follow-up plan, efficacy, and safety profile of the vaccine known yet, etc.
“ICMR has already signed the memorandum of understanding (MoU) with Panacea and the process of signing with SII is already underway,” Dr Gupta said, adding that the manufacturers will also need time to make the vaccine before they can start inoculating the participants in the trial.
“Once manufacturing is done, safety checks are completed, then only trials will begin,” said Dr Gupta, who is heading the project. “Trials may start by October this year.”
Role of ICMR in dengue vaccine project
According to the expression of interest floated by ICMR, it will offer the institutional infrastructure for undertaking further research and development activities and vaccine clinical trials for evaluation of efficacy along with safety and immunogenicity as per regulatory requirements.
ICMR, in March, had invited the expression of interest from the Indian manufactures who have developed potential tetravalent vaccine candidate/or have a non-exclusive licence of the tetravalent vaccine candidates and intend to conduct field study as phase 3 clinical trial after completion of phase 1 and phase 2 trials for successful development of a dengue vaccine.
The collaboration with the manufacturers will be on a royalty basis for a fixed-term contract condition for conducting phase 3 clinical trials.
Under the project, ICMR would provide technical support through a team of experienced scientists in study planning, development of a clinical trial protocol, implementation of phase 3 clinical trial, apart from generating results, data analysis, outcome assessment, safety, immunogenicity, and efficacy assessment. It will also play a role in product improvement and funding essential activities.
Read all the Latest India News here

















Comments
0 comment