views
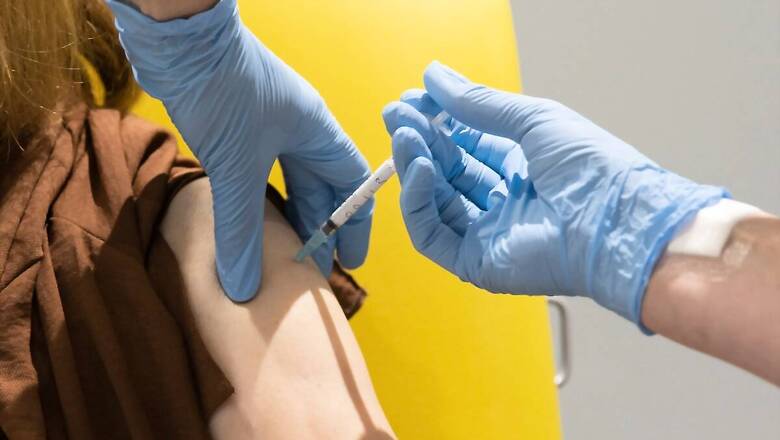
Novavax, the little-known Maryland company that received a $1.6 billion deal from the federal government for its experimental coronavirus vaccine, announced encouraging results in two preliminary studies on Tuesday.
In one study, 56 volunteers produced a high level of antibodies against the virus without any dangerous side effects. In the other, researchers found that the vaccine strongly protected monkeys from coronavirus infections.
Although it’s not possible to directly compare the data from clinical trials of different coronavirus vaccines, John Moore, a virologist at Weill Cornell Medicine who was not involved in the studies, said the Novavax results were the most impressive he had seen so far.
“This is the first one I’m looking at and saying, ‘Yeah, I’d take that,’” Moore said.
Angela Rasmussen, a virologist at Columbia University who was not involved in the studies, called them “encouraging preliminary results,” but cautioned that it won’t be possible to say whether the vaccine is safe and effective until Novavax conducts a large-scale study — known as Phase 3 — comparing people who get vaccinated to people who get a placebo.
The studies are being submitted to scientific journals to be reviewed for publication, said Dr. Gregory Glenn, the president of research and development at Novavax.
The company has said that if its vaccine is shown to be effective, it can produce 100 million doses by the beginning of next year, or enough to give to 50 million people if administered in two doses. Under its deal with the federal government, the company will also receive money to undertake large-scale manufacturing of millions more doses if the vaccine is shown to work.
Novavax’s vaccine is one of more than two dozen products to have entered the first round of safety tests in people, known as Phase 1 trials. Five other coronavirus vaccines are already in Phase 3 trials, in which thousands of people are tested to see if a vaccine works.
But Novavax, which has never brought a vaccine to market in its 33-year history, uses a formula that’s different from all the other vaccines that have produced results in humans so far.
Its vaccines contain a coronavirus protein that prompts a response from the immune system. Protein-based vaccines have a longer track record than some of the newer approaches used by competing coronavirus vaccines, such as those based on viral genes or so-called adenoviruses.
Protein-based vaccines are licensed for diseases such as hepatitis B and shingles. Novavax successfully completed a Phase 3 trial for a protein-based vaccine for influenza earlier this year and has done research on other diseases, such as Middle East respiratory syndrome.
Novavax’s technology turns moth cells into factories for a coronavirus protein called spike, which studs the surface of coronaviruses. Its vaccine combines several of the spike proteins in a nanoparticle.
To improve the performance of the vaccine, Novavax mixed the spike proteins with a compound called an adjuvant. Studies on mice had previously shown that the adjuvant stimulates immune cells so that they develop a potent response to the virus.
The researchers gave the protein and adjuvant to monkeys in different combinations of doses. The monkeys began making high levels of antibodies that could specifically block the coronavirus.
When the monkeys were infected, some versions of the vaccine left them with no trace of the vaccine in their lungs or noses.
“That’s pretty remarkable,” said Akiko Iwasaki, an immunologist at Yale University. She noted that the Novavax vaccine provided stronger protection for monkeys than have other coronavirus vaccines, such as Moderna’s messenger RNA vaccine.
In May, Novavax started a Phase 1 human trial on 134 volunteers. Some of the people who received the vaccine experienced tenderness at the spot where they got injected. But the researchers found no serious side effects.
The researchers extracted serum from the vaccinated volunteers and mixed it with coronaviruses and cells. This showed that the volunteers produced high levels of antibodies that prevented the viruses from infecting cells. The vaccine produced more antibodies in the volunteers than in patients who had recovered from COVID-19 on their own.
“There’s no way to know yet what level leads to protection,” said Matt Frieman, a virologist at the University of Maryland School of Medicine and a co-author of the Phase 1 study. “But all of these pieces point to it being quite effective.”
Volunteers who only received the spike protein in their vaccine did not make a lot of antibodies, the researchers found. “The adjuvant is critical,” Glenn of Novavax said.
Moore said that the volunteers’ strong response to the vaccine “does not surprise me in the slightest.” In June, he and his Weill Cornell colleague P.J. Klasse predicted protein-based vaccines would produce a strong antibody response in a review they published in the Journal of Virology.
Although Novavax is the first company to share clinical results on the immune response of a protein vaccine, it is not the only one testing this technology. Three other protein-based vaccines for COVID-19 — from Clover Biopharmaceuticals, the University of Queensland and Vaxine — are also in Phase 1 trials.
The World Health Organization lists more than 50 protein-based coronavirus vaccines in preclinical trials. One of them is being developed by Sanofi and GlaxoSmithKline, a partnership that last month was granted $2.1 billion from the federal government for 100 million doses. Sanofi expects to start its Phase 1 trial next month.
Sanofi and Novavax both manufacture their vaccines inside the cells of the fall armyworm moth, which allows them to be produced more quickly than older methods that use mammal cells. This technique is one reason Novavax’s vaccine candidate has gotten so much attention — in addition to its deal with the U.S. government, the company has also secured up to $388 million from the nonprofit Coalition for Epidemic Preparedness Innovations, which seeks to make vaccines available outside of the United States. Sanofi’s Flublok vaccine, which is already on the market, uses this technology.
Immunologists have been debating the importance of antibodies for fighting the coronavirus. It’s possible that another part of the immune system, T cells, is also needed to fend off the virus. Moore speculated that the best vaccine against COVID-19 might marshal both types of responses.
A protein vaccine could provide strong antibody protection, while another vaccine — perhaps one based on messenger RNA or an adenovirus — could enlist T cells.
“If these first-generation vaccines are safe but they’re just not potent enough, then you would certainly want to look at combining them,” Moore said.
Carl Zimmer and Katie Thomas c.2020 The New York Times Company


















Comments
0 comment