views
Determining Electron Number in Neutral Atoms
Obtain a periodic table of elements. This is a color-coded table that organizes all the known elements by atomic structure. Each element has a 1, 2, or 3-letter abbreviation and is listed along with its atomic weight and atomic number. Periodic tables can easily be found in chemistry books as well as online.
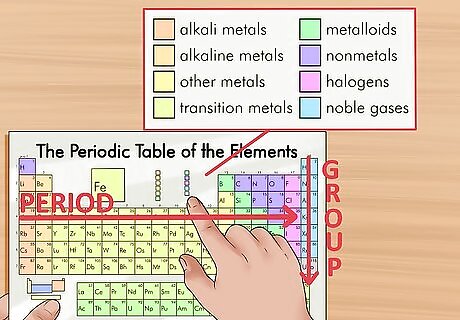
Find the element in question on the periodic table. The elements are ordered by atomic number and separated into three main groups: metals, non-metals, and metalloids (semi-metals). They are further grouped into families including alkali metals, halogens, and noble gases.Every column of the table is called a group and every row is called a period. If you know the details of your element, such as what group or period it is in, it will be easier to locate. If you don’t know anything about the element in question, just search the table for its symbol until you find it.
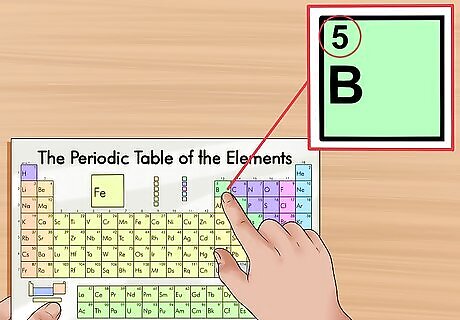
Find the atomic number of an element. The atomic number appears in the upper left-hand corner or centrally above the element symbol in the square. The atomic number defines the number of protons present in that particular element. Protons are the particles in an element that provide a positive charge. Because electrons are negatively charged, when an element is in its neutral state, it will have the same number of protons as electrons. For instance, boron (B) has an atomic number of 5, meaning that it has 5 protons and 5 electrons.
Determining Electron Number of Positively/Negatively Charged Ions
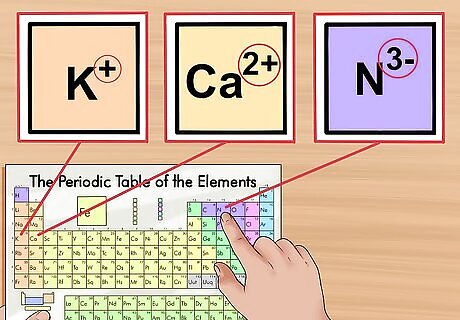
Identify the charge of the ion. Adding and removing electrons from an atom does not change its identity, but it changes its charge. In these cases, you now have an ion, such as K, Ca, or N. Usually, the charge is expressed in a superscript to the right of the atom abbreviation. Because an electron has a negative charge, when you add extra electrons, the ion becomes more negative. When you remove electrons, the ion becomes more positive. For example, N has a -3 charge while Ca has a +2 charge.
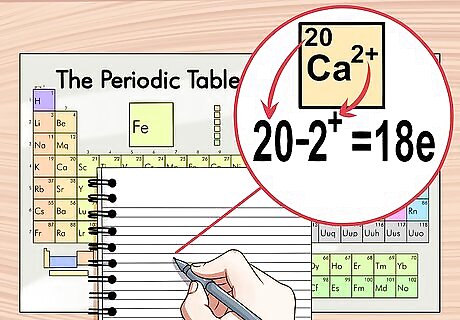
Subtract the charge from the atomic number if the ion is positive. If the charge is positive, the ion has lost electrons. To determine how many electrons are left, subtract the amount of charge from the atomic number. In this case, there are more protons than electrons. For example, Ca has a +2 charge, therefore, it has 2 fewer electrons than a neutral calcium atom. Calcium’s atomic number is 20, therefore this ion has 18 electrons.
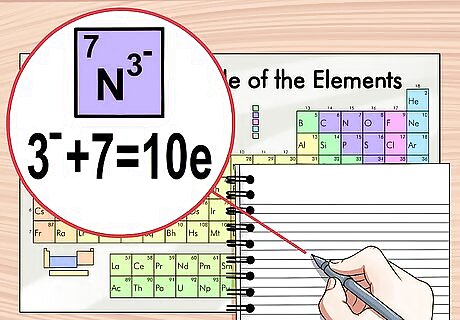
Add the charge to the atomic number if the charge is negative. If the charge is negative, the ion has gained electrons. To determine how many total electrons there are, add the amount of charge to the atomic number. In this case, there are fewer protons than electrons. For example, N has a -3 charge which means it has 3 more electrons than a neutral nitrogen atom. Nitrogen’s atomic number is 7, therefore this ion has 10 electrons.

















Comments
0 comment