views
Making Cuprous Oxide
Cut 2 copper sheets. You can use sheet metal shears to do this easily. Make the sheets the same size. You want 1 sheet to fit on your burner or hot plate, and both to fit in the 2-liter bottle. Making them both 6 in (15 cm) squares should work well.

Clean your copper sheets. Use a degreaser to remove any oils or grease from your copper sheet. You do not want them to react with the copper or to prevent oxidation from happening. You should also wear gloves to avoid getting oil from your skin on the copper. Also be sure to scrub the copper with steel wool or sandpaper to remove any corrosion.
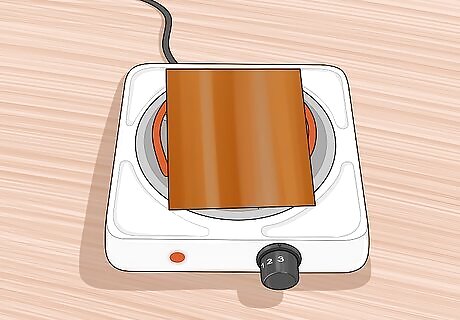
Place 1 copper sheet on a hot plate. Once you have placed a sheet on the hot plate, turn the hot plate on. This will heat the copper and provide the energy needed for the copper to react quickly with the oxygen in the air. This speeds up the natural oxidation process considerably.

Allow the copper to oxidize completely. As the copper heats up you will see varying shades of red, pink, purple, and possibly other colors. This is a sign that oxidation is taking place. Finally, you will notice that these colors are all being replaced by a black covering. This covering is cuprous oxide. Once the whole sheet is covered in cuprous oxide, allow it to cook at least 30 more minutes. Cooking the extra 30 minutes makes the cuprous oxide layer thick and brittle. This allows it to break away from the copper. A thin layer of cuprous oxide would remain on the copper, covering up the cupric oxide layer that needs to be exposed.

Allow the copper to cool slowly. When you are finished cooking the copper, turn the burner off. Leave the copper on the burner to cool. This allows the copper to cool very slowly to room temperature, which should take about 20 minutes. During the cooling process the copper and the cuprous oxide layer shrink at different rates. As long as the layer is thick enough, this will result in the black cuprous oxide flaking off and exposing the red cupric oxide layer. Note that cupric oxide (Copper (II) oxide) is the fully oxidized form, and cuprous oxide (Cu2O) is still in an active state. You can rinse the sheet under water to remove the remaining black deposits. Cupric oxide is a semiconductor and must be exposed in order to make the solar cell function.
Assembling the Solar Cell
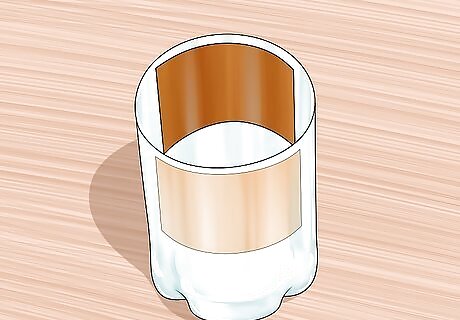
Place the 2 copper sheets into your container. You will need to bend both pieces to match the curvature of the plastic bottle. Both pieces need to be able to fit in the bottle without touching each other. Be sure not to damage the red cuprous oxide layer when bending the cooked sheet.
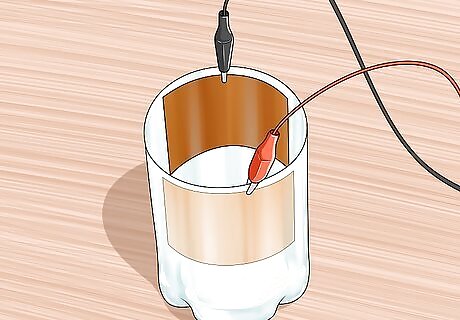
Connect alligator clips to each sheet. Use the alligator clips to attach both pieces to opposite sides of the plastic bottle. The copper sheet with red cuprous oxide should be connected to the clip that will lead to a negative terminal, and the clean copper sheet could be joined to a clip leading to a positive terminal.

Make a saltwater solution. Dissolving salt into the water will provide electrolytes in the form of Na+ and Cl- that carry the current from the cuprous oxide layer to the clean copper sheet. An effective solution will be comprised of roughly one part salt (table salt is fine) to three parts water. Stir well to make sure that the salt is all dissolved. Heat if needed. For example, dissolve ⁄4 cup (59 mL) of salt into ⁄4 cup (180 mL) of water. Using distilled or deionized water will reduce the risk of contaminants.
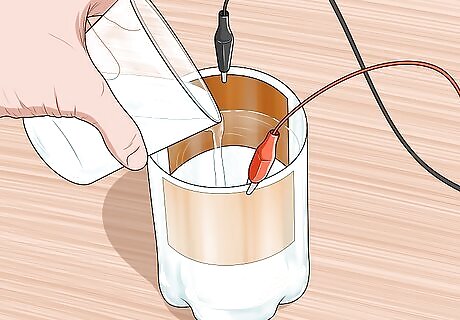
Add saltwater to cover most of the 2 plates. Leave about 2 inches (5.1 cm) of space above the saltwater.This will allow current to travel from the negative terminal to the positive terminal. Be careful to keep the clips at the top of the two sheets dry. Otherwise, the water on the clips might interfere with your readings.
Testing Your Solar Cell
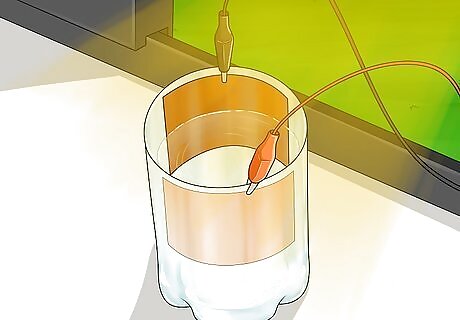
Place the solar cell in the sun. When the sun hits the cuprous oxide layer, it causes electrons to be released. The cuprous oxide is not conductive, but the electrons are able to move through the salt water to the conductive copper plate. This plate transfers the electrons to the wires.
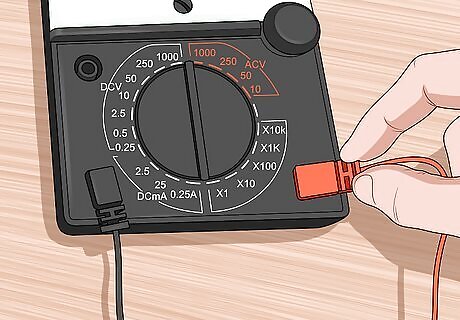
Hook the alligator clips to a multimeter. Plug the other end of your alligator clips into a multimeter or ammeter. Be sure that your meter can function in the microamp (0.000001 amps) range. Plug the positive alligator clip into the positive terminal of your meter and the negative alligator clip into the negative terminal of your meter.
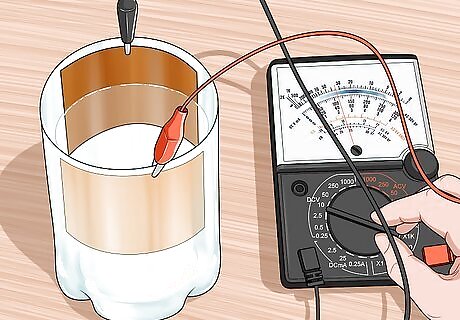
Set your meter to read microamps. A very small amount of current will be flowing. This current should fall somewhere between 0 and 50 microamps. Turning the cell so that the cuprous oxide layer is facing the most direct sunlight will give you the most current.

















Comments
0 comment